2. National Key Lab of Seedline Bioengineering, Yinchuan,750000, P.R. China
 Author
Author  Correspondence author
Correspondence author
Molecular Plant Breeding, 2012, Vol. 3, No. 6 doi: 10.5376/mpb.2012.03.0006
Received: 18 Feb., 2012 Accepted: 03 May, 2012 Published: 04 May, 2012
Liu et al., 2012, Expression of E. coli glgB in Potatoes Generating Tuber Starches with Various Features, Molecular Plant Breeding, Vol.3, No.6 57-62 (doi: 10.5376/mpb.2012.03.0006)
Expression of glgB in amylose-free mutant leads to accumulation of phytoglycogen of low molecular weight in potato tuber. Performance of glgB expression in wild type potatoes is unknown at this point. In this study a glgB was isolated from E. coli JM 109 and it was expressed in two potato genotypes after function of glgB was confirmed. 18 transgenic lines were obtained by PCR and Southern blot screening using glgB transformants. RT-PCR showed that glgB, driven by 35S promoter, was expressed at different levels in 18 transgenic lines. Starch viscosity and amylose contents were assayed with tuber starches of the 18 lines. Data analysis showed that although starch viscosity of all lines was significantly higher than that in the control, genotype of DXY expressing glgB showed much higher starch viscosity than genotype of ZHB expressing glgB did. Meanwhile, amylose contents were the same as those in the control and they were constant in all individuals with ZHB as host expressing glgB. However, all individuals with DXY as host expressing glgB showed a higher amylose contents than those in the control. The results indicated that starchgeneration altered along with different genotypes of transformed potatoes hosting gene. Expression of glgB led to a higher starch viscosity of various extents.
Genetic improvement on the endogenesis starch is rapidly expanding the application of starch. Regulation of downstream gene expression in the starch biosynthesis pathway generated potato starch with shorter chains and less amylose. Study on starches modified by granule-bounded starch synthase (GBSSI) found that the amylopectin cluster and the starch granule remained the same as those in the control, but the structure of the amylopectin cluster changed to form starch with unique potential dynamic values in application (Kozlov et al., 2007).
The potato expressed E.coli glgC increased tuber starch accumulation through raising ADP-glucose pryphosophrylase (AGPase) activity (Stark et al., 1992). Inhibition of GBSSI expression led to reduction in the amylose in the potato starch (Kuipers et al., 1994), and starch branch enzyme (SBE) activities in transgenic lines of which SBE A and B were suppressed were less than 1% of those of the control. Amylopectin with high molecular weight was no longer synthesized, thus starch granule changed morphologically, and phosphorus content in the starch increased by 5 times. This starch is potentially valuable for application (Schwall et al., 2000).
Starch branch enzyme and soluble starch synthase (SSS) are closely related to the apparent amylose content and taste quality in rice. SBE1 and SBE3 express specifically the genotype of high palatability (Sun et al., 2011). SBEIIa is essential for transportation of temporary-storage starch in maize leaves along with the cycle of day and night (Yandeau-Nelson et al., 2011). Activity of ADP-glucose pryphosophrylase in the potato tuber changes with the day-night circle (Tiessen et al., 2002). In addition, the other enzymes involving in starch synthesis including SBE are sensitive to factors such as the day-night circle, which limits generation of different kinds of starches (Jobling, 2002).
Expression of E. coli glgB in amylose-free potato led to generation of tuber starch with low molecular weight and increased branch points (Anne et al., 1996). Amylose is pe-required for formation of alpha 1-6 branch chain in starch; however, much less amyloses were used as substrates in amylose free potato. Branch enzymes, encoded by native potato SBE and E. coli glgB expressed in potato, work with much less amyloses as substrates. This resulted in an unstable reaction catalyzed by the branch enzymes in tubers since both branch enzymes were sensitive to the day and night cycle.
With a relative constant (not much less in amylose potato) supply of amyloses as substrates, shortage of substrates for alpha 1~6 branch as it is in amylose free potato could be avoided. In other words, branch enzyme activity was increased by expression of glgB in wild type potatoes rather than in mutants. Starch molecular weight and other property could be improved, but it is still unknown what aspect of starch was improved when branch enzyme was enhanced in a wild type potato.
In this study, glgB, driven by 35S promoter is expressed in the two wild types of potatoes. It was found that glgB expression showed a genotype specific effect on starch viscosity. Results showed that the starch tuber starch viscosity in glgB transformed lines was 15 time higher than that in the control. Measurement and analysis of the starch indicate that glgB can be expressed in different potato genotypes, which result in different starch types with improved starch viscosities as high as 15 times as the control group.
1 Results
1.1 glgB isolation
With glgB specific primer, a 2 184 bp product was amplified as shownin Figure 1A. The product was ligated into pGEM-T and sequenced. pG-gB+ and BL-gB- were recognized after sequence alignment. With the glgB sequence BLAST was done in the Nucleotide database in NCBI. It was found that glgB sequence isolated from this study is 99% similar to the glgB sequence in the JM109 genome recorded in the database (Welch et al., 2002). Putative polypeptide of the glgB is made up of 728-amino acid. After aligning it with other glgB sequences from other bacterial strains, it was found that there are 3 specific amino acid mutation sites in the putative polypeptide, which are K39E, V71A and I248V. The sequence was registered in NCBI nucleic acid database with accession number EU44744.
Figure 1 glgB isolation and constructing recombinant plasmids |
Digestion of purified pG-gB+ with BamHâ… , Ncoâ… and Sacâ… released a1.3 kb fragment and a 2.2 kb fragment, respectively, as shown in Figure 1B. The same was done with pG-gB-, and the result is shown in figure 1C. All fragments were the same as expected.
1.2 glgB expression in recombinant strains
To ensure glgB with three mutation sites function in glycogen biosynthesis as expected, functional confirmation of the glgB isolates was done by expressing glgB in E.coli. To do so, the glgB was subcloned into downstream of T7 promoter in pET-28c in both 5'-3' and 3'-5' directions. Purified recombined plasmids pE-gB+ and pE-gB- were digested with Xbaâ… and Pstâ… as well as BamHâ… . As shown in Figure 1D and 1E, expected fragments were released from pE-gB+ and pE-gB-. It indicated that the recombined bacterial strains BL-EgB+ and BL-EgB-, containing the pE-gB+ and pE-gB- respectively, were constructed.
To find effect of glgB expression on glycogen in the strains, glycogens from BL-EgB+, BL-EgB- and BL-21 were extracted and scanned with 190-1900 nm wavelength coverage. The result was shown in Figure 2A, 2B and 2C. The absorption peaks of glycogen from BL-EgB+ were the same as that from BL-21, but different from those of BL-EgB-. An extra absorption peak appeared between 300nm and 400nm in glycogen from BL-EgB-, which is absent in other strains. This indicated that the expression of reverse glgB driven by T7 promoter changed the glycogen structure in BL-EgB-. Therefore, it is concluded that glgB isolate is functional in glycogen biosynthesis.
Figure 2 Light-absorption features of glycogen from the recombined bacteria BL-gB+and BL-gB- |
1.3 glgB expression in potato transgenic lines
To express glgB in potatoes, transformation vector pC-SaS was constructed and transformed into agrobacterial LBA4404 to generate recombinant strains LBA-gB. The recombined pC-gB was extracted from LBA-gB, and it was cut with BamHâ… and EcoRâ… . A 1.2 kb and a 940 bp as well as a 2.2 kb fragment were released, respectively, as shown in Figure 3.
.png) Figure 3 Digestion result of vector pC-gB |
26 PCR positive transformants were found from two groups of transgenic lines. Some of those were shown in figure 4A. Southern blot showed that among the 26 PCR positive transformants, 9 were inserted with single copy of the recombined glgB, and the other 9 with double copies of the recombined glgB, as shown in Figure 4B.
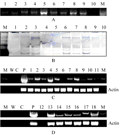 Figure 4 Test of transgenic lines on genome and transcriptome level |
RT-PCR result with RNA of the 18 transgenic lines and glgB specific primers showed that glgB mRNA was expressed in all of 15 transgenic lines. The expression amounts in 4 transgenic lines were much higher than the other 10, as shown inFigure 4C and 4D.
1.4 Stain of starches from transgenic lines
Minitubers, weight between 2 g and 5 g were harvested from 15 transgenic lines: 6 from DXY host lines, and 9 from ZHB host lines. Based on the starch staining, 15 transgenic lines were divided into 2 categories. 8 lines fall into the first category, as starch strained from those lines remained blue, but lighter than their positive control, such as lines of DF5 and DF3 from DXY host, and lines of ZF10 and ZF7 from ZHB host. The other 8 lines are in the second category, as starch strained from those lines turned light blue, such as DF2 and DF6 from DXY host, and ZF6 and ZF8 from ZHB host. Typical color results were partially shown in Figure 5.
 Figure 5 Lugol staining results of the starches from transgenic lines |
1.5 Starch viscosity and amylose content of tuber from transgenic lines
Based on results of starch staining, 8 lines with typical blue and light blue, 4 from DXY host and 4 from ZHB host were selected for analysis of starch viscosity and amylose contents. In the line of DXY genotype expressing glgB, the highest starch viscosity, 388.06 mPa.s occurred in the line of DF6, which was 12 times higher than the control. Starch viscosity of DF3 and DF6 were much higher than DF5 and DF2, and also significantly higher than the control (p<0.01).
In the group with ZHB genotype as the host expressing glgB, the highest starch viscosity, from the line ZF7 was 212.46 mPa.s, which was 15 times higher than the control; viscosity of ZF7 and ZF8 were significantly higher than that of ZF6 and ZF10, as well as the control group (p<0.01), as shown in Figure 6.
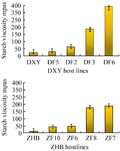 Figure 6 Starch viscosities of the transgenic lines |
Starch viscosity of the 8 transgenic lines varied between 36.12 mPa.s and 388.06 mPa.s.
Data analysis on amylose content (AC) indicated, as shown in figure 7 that AC in DXY host lines was significantly higher than that in control. Among them, the highest AC was in the line DF6 and it was 1.2 times higher than the control. ACs of the 4 transgenic lines from ZHB host were the same as that in the control.
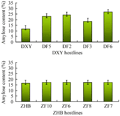 Figure 7 Amylose contents (AC) test result in transgenic lines that express glgB |
The line of the DXY host was different from that of the ZHB host in both starch viscosity and amylose content. Starch viscosity in DXY host line was much higher than that in ZHB host line. Amylose content in DXY host line was significantly higher than the control, but it was the same between ZHB host line and control. Therefore, glgB expression in different host genotypes led to various responses for starch biosynthesis.
Data analysis showed that there was no significantly correlation between starch viscosity and amylose contents of the transgenic lines. There wasn’t any correlation between amount of RT-PCR products and starch viscosity, either. Difference in starch viscosities between two genotypes host lines and the controls indicates that glgB expression within potato regulates starch biosynthesis through increasing amylopectin synthesis. This result is in accordance with results obtained when glgB is expressed in amf potato done by Anne et al (1996). Meanwhile, effect of glgB expression in potato on amylose biosynthesis shows genotype specificity.
2 Discussions
In this study, we transformed glgB into two potato cultivars. Two glgB -expressing transgenic groups were obtained. Starch viscosity and amylose content in starches isolated from the two groups were assayed. Data analysis of the assay indicated that two genotypes performed differently in response to glgB expression. This is the first evidence to show crop genotype-specific response to an extraneous gene expression. This means that more than one crop genotypes have to be taken into consideration when a transgenic breeding program is made to improve starch quality of the crop.
In addition, in this study, we found that starch viscosities of all transgenic lines were significantly higher than that in the control, one genotype expressing glgB showed much higher starch viscosity than the other genotype expressing glgB. This finding indicates clearly that starch feature can be modified in wider extent than expected through expression of extraneous genes involved in polysaccharide biosynthesis. Plant starch feature could be subjected to not only endogenesis genes but also extraneous relatives.
Many factors could affect the expression of a target gene transformed into a plant species, such as the location of insertion in host genome, completeness of gene inserted, copy number etc; however, alteration of gene expression involving starch biosynthesis, such as starch branch enzyme is a way to find how starch biosynthesis is regulated. It is particularly important for polyploidy starch plant, such as potato; However, it can never be neglected that even though glgB is completely inserted and expressed, alteration of starch biosynthesis resulting from glgB may be different in different genotypes of a plant, because initiation and regulation of starch accumulation in the early stage is variable among different genotypes. Therefore, a further study on the transgenic lines is needed to reveal variation in stabilized accumulated starches.
Starches used in this study were isolated from immature tubers, which are different from the full mature tuber; therefore, immature tubers may be different in the starch composition from mature tubers. Though non-transformed genotypes grown under the same condition are regarded as the control in comparing results of starch test in the study, further assessment on the starch features in different transgenic lines is needed when mature tubers are harvested outdoor.
3 Material and Methods
3.1 glgB isolation
Genome DNA was isolated following instruction as described on Molecular cloning Molecular Cloning: A Laboratory Manual (Third Edition) (CSH Press 2001). According to E. coli JM109 glgB DNA sequence (AEO16768) in Genbank, primer pairs PF1 and PR1 were designed. PF1: 5'-cagttccatggggtgacacaat-3', and PR1: 5'-ccctgactagtcgtaatgc-3'. With diluted JM109 genome DNA and the primers, 25 μL PCR reaction was set as 10 × PCR buffer solutions (2 μL 10 mM dNTP, 1 µL 1 μmol/L PF1, 1 µL 1 μmol/L PR1, 0.25 μL pfu DNA polymerase). Sterilized DDW was added to fill it up to 25 µL. After mixing them, PCR amplification was performed.
The reaction was carried out as following: denaturation at 95℃ for 30 sec, annealing at 52℃ for 60 sec, extension at 72℃ for 3.5 min, (30 cycles in total) extension at 72℃ for 10 min and keeping it at 4℃ for the last cycle. PCR products were recovered with a gel recovery kit (Promega).
To set up the ligation reaction of PCR products and vector, a reaction was done with 3 µL recovered PCR products and 1 µL pGEM-T easy vector following instruction of the pGEM-T easy kit. 2 µL ligation reaction products were transformed into E. coli supercompetent cells by heat shock. Transformed cells were plated on a LB patri dish. Cells were grown overnight. The white clones were screened. Positive clones were selected by Ncoâ… and Sacâ… digestion analysis and confirmed by sequencing (ABI 3730XL DNA Sequencer at the Shenggong, Shanghai, China). Target recombined bacterial strains BL-gB+ and BL-gB-, containing glgB in 5'-3' (pG-gB+) and 3'-5' (pG-gB-) direction, respectively were obtained.
3.2 Construct of pE-gB
Isolate recombinant plasmid pG-gB+ and pE-GB. Cut both DNA with Ncoâ… and Sacâ… . Recovery and purify cut fragments. Ligate the fragment with T4 DNA ligase and transform it into the super competent cells of host bacteria BL21. The recombined plasmid pE-gB+, in which glgB was downstream of the T7 promoter was obtained. Likewise, the recombined plasmid pE-gB- was obtained. Recombined bacterial strains BL-EgB+ and BL-EgB-, containing pE-gB+ and pE-gB- respectively were proven by digestion analysis with Ncoâ… and Sacâ… as well as BamHâ… . Glycogen from recombined bacterial strains was isolated and measured in accordance with methods used by Haugen et al (1976).
3.3 Construction of pC-gB
Isolate pC-SaS from the Agrobacterial LBA4404-SaS, pG-gB from the recombined bacterial strain BL-gB+. Cut pC-SaS and pG-gB with NcoI and SpeI. Recovery and purify 11 kb fragment from pC-SaS and 3.2 kb fragment from pG-gB with a recovery kit (Promega). Ligate fragments with T4 DNA ligase (Promega) and transform ligated plasmid into the competent cells of the E. coli BL5α. After proving glgB being ligated downstream of 35S promoter in pC-SaS, transform the ligated plasmid into competent cells of the agrobacterial to obtain recombined Agrobacterial LBA-gB containing recombined pC-gB.
3.4 Transformation of glgB into potato
Cut 1 cm stem segments with axillary bud from genotype DXY and ZHB as the explants, and pre-culture on MS media for 2 days. According to the protocol (Anne et al., 1994), after plantlet in glass tuber grows up to 8~10 cm at height, transfer it into the peat soil and grow for 30 days under light density of 20 000 Lx, 25℃ and with 12 h irradiation per day. Then move and grow plantlets under the same condition but with 8h irradiation per day. Minitubers were harvested after 30 days.
3.5 Screening for glgB transgenic lines
Isolate genome DNA using CTAB method with leaves from two groups of transformants. With glgB primer cloned from the JM109 genome DNA, PCR was done as described above. PCR product was tested with 1% agarose gel electrophoresis.
glgB probe was made as instruction DIG labeled kit (Mylab, Ltd. Beijing). Southern blot was carried out following Molecular Cloning: A Laboratory Manual (Third Edition) (CSH Press 2001). Hybridization signal on HyBond N+Membrane was tested with Dig Hybridization assay kit (Mylab, Ltd. Beijing). Image of signal on the membrane was taken with digital cameral.
3.6 RT-PCR test of glgB expression in transgenic lines
Leaf DNA was extracted as described above from each of the transgenic lines. With glgB prime as above, RT-PCR was done as the instruction of RT-PCR kit (Takara).
3.7 Staining starch from the transgenic lines
Extract starch with method as described by Kuipers et al (1994), 0.02% starch suspension at 20℃ was stained with 5% Lugol.
3.8 Assay of amylose contents
Assay of amylose contents was done with the method as described by Kuipers et al (1994).
3.9 Assay of starch viscosity
To measure the dynamic viscosity of tuber starch, the following reactions were made: add 3 g of purified starch (water contents 16%) in ddH2O to make 3% water suspension(w/v).Heat up suspension to 70℃ and incubate at 70℃ for 15 min for gelatinization. Add 10 mL the gelatinized suspension to a capillary viscometer in a device kept constant at 60℃. The time required for the suspension to fully pass a 3 mm interstice in the viscometer was recorded as t1. Dynamic viscosity was calculated with the formula η=ηr×t1/t0. ηr is the solvent viscosity, and here ηr= 0.469 mPa.s when ddH2O goes through the 3 mm interstice at 60℃, t0 is the time required for equal volume solvent (ddH2O) to flow through the interstice. All data was analyzed by a statistic tool, DPS ver. 9.50 (Data Processing System, Ruifeng, Hangzhou, China).
Authors' Contributions
YXL designed the experiment andprimers used, performed data analysis, wrote and revised the manuscript and supervised performance of the project. LJF performed glgB isolation, vector construction and glgB function confirmation in E. coli. FXJ performed glgB transformation into potatoes, screening for transgenic lines expressing glgB and phenotype assay. CXQ, CQ and HQ involved partially in the experiments above.
Acknowledgements
This work was jointly supported by National Sci-Tech Support Program in China (2009BADC5B01, to Yao XL) and National Natural Science Foundation of China (NSFC 30660079, 31160236, To Yao XL).
References
Anne J.K., Angela M.S., Vermeesch B.J., de Vries, Evert J., and Richard G.F.V., 1996, Expression of Escherichia coil branching enzyme in tubers of amylose-free transgenic potato leads to an increased branching degree of the amylopectin, The Plant Journal, 10(1): 83-90
http://dx.doi.org/10.1046/j.1365-313X.1996.10010083.x PMid:8758980
Haugen T.H., Ishaque A., and Preiss J., 1976, Biosynthesis of bacterial glycogen, characterization of the subunit structure of Escherichia coli B glucose-1-phosphate adenylyltransferase (EC 2.7.7.27), The Journal of Biological Chemistry, 251(24): 7880-7885
PMid:826540
Jobling S.A., Westcott R.J., Tayal A., Jeffcoat R., and Schwall G.P., 2002, Production of a freeze-thaw-stable potato starch by antisense inhibition of three starch synthase genes, Nat Biotechnol, 20(3): 295-299
http://dx.doi.org/10.1038/nbt0302-295 PMid:11875432
Kozlov S.S., Blennow A., Krivandin A.V., and Yuryev V.P., 2007, Structural and thermodynamic properties of starches extracted from GBSS and GWD suppressed potato lines, International Journal of Biological Macromolecules, 40(5): 449-460
http://dx.doi.org/10.1016/j.ijbiomac.2006.11.001 PMid:17188347
Kuipers G.J., Jacobsen E., and Visser R.G.E., 1994, Formation and deposition of amylose in the potato tuber starch granule are effected by the reduction of granule-bound starch synthase expression, The Plant Cell, 6(1): 43-52
http://dx.doi.org/10.1105/tpc.6.1.43 PMid:12244219
http://dx.doi.org/10.2307/3869673 PMid:12244219 PMCid:160414
Tiessen A., Hendriks J.H.M., Stitt M., Branscheid A., Gibon Y., Farré E.M., and Geigenberger P., 2002, Starch synthesis in potato tubers is regulated by post-translational redox modification of adp-glucose pyrophosphorylase, Plant Cell, 14(9): 2191-2213
http://dx.doi.org/10.1105/tpc.003640 PMCid:150765
Stark D.M., Timmerman K.P., Barry G.E., Preiss J., and Kishore G.M., 1992, Regulation of the amount of starch in plant tissues by ADP gtucose pyrophosphoryiase, Science, 258(5080): 287-292
http://dx.doi.org/10.1126/science.258.5080.287 PMid:17835129
Schwall G.P., Safford R., Westcott R.J., Jeffcoat R., Tayal A., Shi Y.C., Gidley M.J., and Jobling S.A., 2000, Production of very-high-amylose potato starch by inhibition of SBE A and B, Nat Biotechnol, 8(5): 551-554
Sun M.M., Abdula S.E., Lee H.J., Cho Y.C., Han L.Z., Koh H.J., and Cho Y.G., 2011, Molecular aspect of good eating quality formation in Japonica rice, PLoS One, 6; 6(4): e18385
Yandeau-Nelson M.D., Laurens L., Shi Z., Xia H., Smith A.M., and Guiltinan M.J., 2011, Starch-branching enzyme IIa is required for proper diurnal cycling of starch in leaves of maize, Plant Physiol, 56(2): 479-90
http://dx.doi.org/10.1104/pp.111.174094 PMid:21508184 PMCid:3177252
Welch R.A., Burland V., Plunkett G.D.III., Redford P., Roesch P., Rasko D.A., Buckles E.L., Liou S.R., Boutin A., Hackett J., Stroud D., Mayhew G.F., Rose D.J., Zhou S., Schwartz D.C., Perna N.T., Mobley H.L.T., Donnenberg M.S., and Blattner F.R., 2002, Extensive mosiac structure revealed by the complete genome sequence of uropathogenic Escherichia coli, Proc. Natl. Acad. Sci., U.S.A., 99(26): 17020-1702
http://dx.doi.org/10.1073/pnas.252529799 PMid:12471157 PMCid:139262
. PDF(886KB)
. FPDF
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Jinfeng Liu
. Xiujuan Feng
. Xueqiong Cui
. Qian Cai
. Xinling Yao
Related articles
. Potato
. glg B
. Starch
. Expression
. Genotype
Tools
. Email to a friend
. Post a comment


