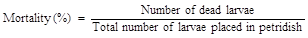Centre for Plant Breeding and Genetics, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India

Author

Correspondence author
Molecular Plant Breeding, 2014, Vol. 5, No. 5 doi: 10.5376/mpb.2014.05.0005
Received: 21 Apr., 2014 Accepted: 10 May, 2014 Published: 28 May, 2014
Vinodhana et al., Development of Insect Resistant Transgenic Lines Mediated by Agrobacterium in Cotton (Gossypium hirsutum L.), Molecular Plant Breeding, 2014, Vol.5, No. 5 24-28 (doi: 10.5376/mpb.2014.05.0005)
Cotton has attracted much interest in the field of genetic engineering and gene transfer for the development of transgenic plants with economically important new traits. The main goal of gene transfer in cotton is the development of bollworm resistant varieties especially with Bt crystal toxin producing (cry) genes. Considerable progress so far made in developing transgenic cotton for bollworm resistance utilized the Bt genes cry1Ab and cry1Ac (Perlak et al., 1990), cry1Ac (Benedict et al., 1996), cry1Ac and cry2Ab (Stewart and Knighten, 2000), cry1Ac (Kategari et al., 2007) and cry1Ec (Kumar et al., 2009). The end goal of Bt technology is to obtain a durable protection, which requires in particular the stability of gene expression during the course of selfing or back crosses and also requires reducing the probability of appearance of resistant insects. However, the potential of insects to evolve resistance against Bt toxins is a serious threat to this technology. To combat from this problem, cry2A genes are considered as promising candidates for management of insects in crop plants owing to their difference in structure and insecticidal mechanism (Jain et al., 2006).
Of the gene delivery systems, Agrobacterium mediated transformation via somatic embryogenesis remains the method of choice for the development of transgenic cotton because of single cell origin of the somatic embryos thus reducing the chimeric transformation events (Khan et al., 2010). However, the recalcitrance of elite cotton genotypes to regeneration through somatic embryogenesis hinders the use of gene transfer technology to improve this crop. As only Coker varieties were found to respond better for gene transfer, most of the desirable genes are introduced initially into Coker and backcrossed into other varieties later. The objective of the study was to transform the Gossypium hirsutum cultivar Coker 310 with cry2Ab gene using the Agrobacterium system for enhancing resistance to Lepidopteran insects.
1 Results and Discussion
Agrobacterium-mediated transformation was carried out usingcry2Ab geneinfive months oldembryogenic calli, since embryogenic calli provide a large population of embryogenic competent cells that are extremely amenable for transformation by Agrobacterium.
In the transformation studies, the embryogenic calli was first subjected to kanamycin sensitivity test to standardize the concentration of kanamycin for effecting the selection of transformed callus. Kanamycin selection for transgenic tissue using the npt II gene as a selectable marker is well-known for cotton and has been commonly used in cotton gene transformation (Wilkins et al., 2004; Khan et al., 2010). Plant cells transformed with the npt II gene can detoxify the antibiotics in the selection medium. In this study, the embryogenic calli when cultured on the medium containing different concentrations of kanamycin (0, 5 mg/L, 10 mg/L, 15 mg/L, 20 mg/L, 25 mg/L, 30 mg/L, 35 mg/L and 40 mg/L) none of the calli survived beyond the concentration of 20 mg/L (Table 1). Therefore, 25 mg/L concentration of kanamycin was chosen for the selection of transformed tissues in transformation experiments.
.png)
Table 1 Effect of different concentrations of kanamycin on the survival of Coker 310 embryogenic calli
|
Seven days old pre-cultured embryogenic calli after cocultivation with Agrobacterium for 72 h, were transferred on to the selection medium containing 25 mg/L kanamycin. During the first round of selection, the co-cultivated calli became dark brown or black. The callus remained dark brown or black also during second selection. Fresh growth was observed from the kanamycin resistant calli in the third selection. After four rounds of selection (each round with 15 days duration), the kanamycin resistant embryogenic calli were at an average frequency of 37.0%. Further two subcultures (each with one month duration) in the same media the kanamycin resistant embryogenic calli resulted in the formation of somatic embryos at an average frequency of 23.7%. The matured cotyledonary stage embryos (3~5 mm size) were germinated on the plantlet development medium without kanamycin selection. Out of 500 embryogenic calli cultured, eight plants were regenerated at a regeneration frequency of 1.6%. All the eight plants were transferred to glass house after hardening.
The eight putative transgenic plants (T0) were confirmed for the presence ofnptII and cry2Ab gene using specific primers. PCR analysis resulted in the expected sizes of 800bp for the nptII (Figure 1) and 611 bp (Figure 2) for cry2Ab gene in five putative transgenic plants. Thus, out of 500 explants co-cultivated, 5 plants were found to be positive at the end that resulted in the transformation efficiency of 1.0%.
.png)
Figure 1 PCR amplification of npt ll gene in putaive transgenic lines of Coker 310
|
.png)
Figure 2 PCR amplification of cry2Ab gene in putaive transgenic lines of Coker 310
|
Allthe five PCR positive plants were subjected to feeding by neonate larvae of the bollworm Helicoverpa armigera. Bioassay on detached leaves showed considerable variation among the transgenic plants. The larval mortality percentage of five T0 transgenic plants ranged from 33.0% to 67.0% (T0 plant no: 3) (Table 2).
.png)
Table 2 Insect bioassay of transgenic cotton plants (T0) against Helicoverpa armigera
|
The larvae fed on the transgenic plants were stunted in growth when compared to larvae fed on non-transformed control plant (Figure 3). Biotoxicity assays conducted by Bakhsh et al. (2009) in transgenic cotton leaves with 2nd instar Heliothis larvae exhibited variable mortality rate of 60%~100%.
Variability in larval mortality and growth rate reduction observed among transgenic cotton plants might be due to the differences in the expression levels of gene in these plants. In this study, however, 100% mortality has not been observed in the transgenic plants analyzed. This could be because of lower level of expression of the cry2Ab gene in transgenic plants. However, with large number of transformation experiments, it would be possible to obtain large number of independent transgenic events.
2 Materials and methods
2.1 Induction of embryogenic calli
Delinted seeds of Coker 310 were surface sterilized with 70% ethanol for 1 min and washed thrice with sterile distilled water. They were again surface sterilized with 0.1% (w/v) aqueous mercuric chloride solution for 10 min and washed thrice subsequently with sterile distilled water and soaked overnight to soften the seed coats. Seed coats were removed next day and the sterile seeds were germinated on a half strength MS (Murashige and Skoog, 1962) basal medium supplemented with 15 g/L sucrose and 9 g/Lagar. The germination bottles were then incubated for one week in the culture room at 25°C±2°C under 16/8 hr photoperiod. Cotyledon (~15 mm2) sections were excised from seven day old cotton seedlings and inoculated in the MS medium supplemented with 0.1 mg/L2, 4-D and 0.5 mg/L kinetin, 30 g/Lmaltose solidified with 4 g/L phytagel for callus induction. After one month of culture (total age of callus –30 days) light-yellow fresh callus were separated from the explant and subcultured in the same medium for another one month for callus proliferation. Highly proliferating callus (total age of the callus -60 days) were transferred to basal MS medium and maintained for two months in the same medium to achieve callus maturation. After callus maturation (total age of callus -120 days) good proliferating callus were transferred to MS medium supplemented with 1.9 g/L KNO3, 30 g/L maltose solidified with 4 g/L phytagel [referred to as somatic embryo induction (SEI) media] for the induction of embryogenic callus. After one month of culture (total age of callus -150 days), vigorously growing, friable, loose and white embryogenic calli were used for transformation experiments.
2.2Kanamycin sensitivity test
To determine the optimum concentration of kanamycin to be used for in vitro selection of transformed tissues, five month old embryogenic calli of Coker 310 was cultured in the SEI mediumsupplemented with different concentrations of kanamycin (0, 5 mg/L, 10 mg/L, 15 mg/L, 20 mg/L, 25 mg/L, 30 mg/L, 35 mg/L and 40 mg/L). The frequency of survived explants was calculated after four rounds of selection (15 days per round of selection). After assessing sensitivity of the explants, kanamycin concentration of 25 mg/L was used for selection of transformed tissues in transformation experiments.
2.3 Transformation with cry2Ab gene
A binary construct harboring the cry2Ab gene under the control of the cauliflower mosaic virus 35S (CaMV 35S) promoter and the plant selectable marker neomycin phosphotranferase (nptII) gene was used for transformation (Figure 4).
.png)
Figure 4 Physical map of pBI121 harbouring cry2Ab and nptll genes
|
Five months old embryogenic calli (derived from 7 days old cotyledon explants) of Coker 310 were precultured in the fresh SEI mediafor different durations (1, 7, 14, 21 days) before Agrobacterium infection. Embryogenic calli with different preculture periods were infected with the bacterial suspension with intermittent shaking for 20 min. The bacterial suspension was removed by pipette and the calli were blotted dry and transferred to Whatman no filter paper (cat no. 1004070) placed on co-cultivation medium composed of SEI media and 100mM acetosyringone. After co-cultivation at 24°C in the dark for 72h, the infected embryogenic calli were transferred to selection medium containing SEI media, 25 mg/L kanamycin and 500 mg/L cefotaxime. After four rounds of selection (each with 15 days duration) in the selection medium, the frequency of kanamycin resistant embryogenic calli was recorded for the callus oBtained from different preculture period (total age of the callus from callus initiation was 7 months). Of the different preculture periods, the embryogenic calli precultured for 7 days were used for further somatic embryo induction studies. For the induction of somatic embryos, the rapidly proliferating kanamycin resistant embryogenic calli were subcultured on fresh selection medium for further two months (each with one month duration) (total age of the callus from callus initiation was 9 months) and the frequency of somatic embryos formed were recorded.
2.4 Plantlet regeneration
The matured cotyledonary stage embryos (3~5 mm size) oBtained were germinated on the plantlet development medium containing full strength MS supplemented with 30 g/L sucrose, 1.0 mg/L ets reached 4~6 leaves stage with sufficient roots (30~45 days of culture in plantlet development media) they were gently removed from the medium and washed in running tap water carefully to remove the media adhering to roots. They were then transferred to paper cups containing a 1:1:1 (vol/vol) mixture of sterilized planting substrates like soil: sand: compost. The plants were covered with polyethylene bags to maintain enough humidity and kept in culture room for 15 days. After 15 days, polypropylene bags were removed and well established plantlets were transferred to glass house.
2.5 PCR analysis
To verify the presence of nptII and cry2Ab genes, PCR was performed with thegenomicDNA samples of putative transgenic and non-transgenic (control) plants using gene specific primers. Reactions were performed in a final volume of 25 μl and the mixture contained 50 ng of genomic DNA, 2.5 μl of 10× PCR buffer (10 mM Tris-HCI (pH 9.0), 50 mM KCl, 1.5 mM MgCl) 200mM of each of dNTPs, 70 ng of upstream and downstream primers and 2 units of Taq DNA polymerase. Amplification was performed in a MYCycler, (Biorad,USA). The primer pair used to amplify a 800 bp long internal fragment of the nptII gene were 5'ATG ATT GAA CAA GAT GGA TTG CACG 3' (Forward primer); 5' TCA GAA GAA CTC GTC AAG AAG GC 3' (Reverse primer). Temperature profile used for amplification of nptII gene was as follows: Pre-incubation at 94°C for 5 min leading to 30 cycles of melting at 94°C for 1 min, annealing at 55°C for 1 min, and synthesis at 72°C for 1 min followed by an extension at 72°C for 15 min. The primer pair used to amplify a 611 bp–long internal fragment of the cry2Ab gene were 5' CTG AGC TCA CGG GTC TGC AAG C 3' (Forward primer); 5' GTT GGA GAG CCG AGC ACC ACT G 3' (Reverse primer). Temperature profile used in the amplification consisted of pre-incubation at 94°C for 5 min leading to 35 cycles of melting at 94°C for 1 min, annealing at 65°C for 45 sec and synthesis at 72°C for 45 sec followed by an extension at 72°C for 10 min. After amplification, 10 µl of the product was used for electrophoretic analysis on 1.0% agarose gels and detected by ethidium bromide staining and photographed under ultraviolet light.
2.6 Insect bioassay
Bioassay was carried out with the leaves of T0 plants and non-transformed (control) plants using the neonate larvae of Helicoverpa armigera. Leaves collected from the putative transgenic and non-transformed (control) plants were placed on a wet filter paper in sterile petridishes. Five larvae were released into each petridish and observations were made every 24 h on the mortality rate of the larvae. After 3 days, insect mortality per cent was calculated as follows
2.7 Statistical analysis
The experiment was carried out in a completely randomized design. Data were analyzed using standard ANOVA procedures and the difference between the treatments means were compared using the Fisher’s Least Significant Difference test (LSD).
Author contributions
There is a paucity of research investigations in the life of people and plants. I have made a very small and humble attempt to reduce a fraction of it. I wish to share my contributions as an author of this research report. The main goal of gene transfer in cotton is the development of bollworm resistant varieties especially with Bt crystal toxin producing (cry) genes. As only Coker varieties were found to respond better for gene transfer, most of the desirable genes are introduced initially into Coker and backcrossed into other varieties later. The objective of the study was to transform the Gossypium hirsutum cultivar Coker 310 with cry2Ab gene using the Agrobacterium system for enhancing resistance to Lepidopteran insects. Good results were obtainedwhich have been interpreted in this research report.I also express the depth of my sense of gratitude to my advisory committee members (co-authors 2 and 3) for their indefatigable guidance, explicit and unaccountable help rendered for this investigation.
Acknowledgement
We gratefully acknowledge ICGEB, New Delhi for providing us the Agrobacterium strain LBA4404 harbouring plasmid pBI121 (containing the cry2Ab gene) for the purpose of sole scientific research.
Bakhsh A., Rao A.Q., Shahid A.A., Husnain T., and Riazuddin S., 2009, "CaMV35S is a Developmental Promoter Being Temporal and Spatial in Expression Pattern of Insecticidal Genes (Cry1Ac & Cry2A) in Cotton", Res. J.Cell Mol Biology, 3: 56-62
Benedict J.H., Sachs E.S., Altman D.R., Ring R.J., and Berberich B.A., 1996, Field performance of cotton expressing cry1A insecticidal crystal protein for resistance to Heliothis virescens and Helicoverpa zea, J. Econ. Entomology, 89: 230-238
Jain D., Udhayasuriyan V., Arulselvi P.I., Dev S., and Sangeetha P, 2006, Cloning, characterisation and expression of a new cry2Ab gene from Bacillus thuriengiensis strain 14-1, Applied Biochemistry and Biotechnology, 128: 185-194
http://dx.doi.org/10.1385/ABAB:128:3:185
Khan T., Reddy V.S., and Leelavathi S., 2010, High-frequency regeneration via somatic embryogenesis of an elite recalcitrant cotton genotype (Gossypium hirsutum L.) and efficient Agrobacterium-mediated transformation, Plant Cell Tiss Org., 101: 323-330
http://dx.doi.org/10.1007/s11240-010-9691-y
Kumar M., Shukla AK., Singh H., and Tuli R., 2009, Development of insect resistant transgenic cotton lines expressing cry1EC gene from an insect bite and wound inducible promoter. J. Biotechnol., 140: 143-148
http://dx.doi.org/10.1016/j.jbiotec.2009.01.005
Leelavathi S., Sunnichan S.G., Kumria R., Vijayakanth G.P., Bhatnagar R.K., and Reddy V.S. 2004, A simple and rapid Agrobacterium-mediated transformation protocol for cotton (Gossypium hirsutum L.), Embryogenic calli as a source to generate large numbers of transgenic plants, Plant Cell Rep., 22: 465-470
http://dx.doi.org/10.1007/s00299-003-0710-x
Perlak F.J., Deaton R.W., Armstrong T.W., Fuchs R.L., Sims S.R., Greenplate J.T., and Fischoff D.A., 1990, Insect resistant cotton plants, BioTechnol., 8: 939-943
http://dx.doi.org/10.1038/nbt1090-939
Stewart S.D., and Knighten K.S., 2000, Efficacy of Bt cotton expressing two insecticidal proteins of Bacillus thuringiensis kurstaki on selected caterpillar pests, pp.1043-1048, In: Dugger P. and Richter D. (eds.). Proceedings Beltwide Cotton Conference National Cotton Council of America, Memphis, Tennesse, USA
Wilkins T., Mishra R., and Trolinder N.L., 2004, Agrobacterium mediated transformation and regeneration of cotton, J. Food, Agric Environ, 2: 179-187

 Author
Author  Correspondence author
Correspondence author
.png)
.png)
.png)
.png)