 Author
Author  Correspondence author
Correspondence author
Molecular Plant Breeding, 2015, Vol. 6, No. 5 doi: 10.5376/mpb.2015.06.0005
Received: 26 Dec., 2014 Accepted: 31 Jan., 2015 Published: 25 Feb., 2015
Nourredine et al., Changes of peroxidase activities under cold stress in annuals populations of Medicago, Molecular Plant Breeding, 2015, Vol.6, No. 5 1-9 (doi: 10.5376/mpb.2015.06.0005)
Up to 15 percent of the world’s agricultural production is lost to frost. Increasing knowledge on antioxidant systems under chilling stress could lead to understanding tolerance to extreme temperatures and at least lead to possible genetic improvements. To minimize oxidative damage, plants have evolved various enzymatic and non-enzymatic defense mechanisms to detoxify free radicals and reduce oxidative stress. Changes of peroxidase activities are generally related to cold stress responses. The aims of this work was to investigate changes in antioxidant enzyme peroxidase EC 1.11.1.7 (POD) activities of 8 accessions of Medicago exposed during post germination to low non-freezing temperature. After 3 days of germination, levels of enzyme activity were examined at different durations, 5, 8, and 11 days at a low temperature (4°C) (T1, T2,and T3) and untreated plantlets (controls), 2, 4, 6, 8, 10, 11, 12, and 14 days at 22°C (T02, T04, T06, T08, T010, T011, T012,and T014) respectively. The most noticeable result in this current study, POD activities were higher under low temperature regimes than control. This activities increases in the beginning of the stress in both the tolerant and the sensible accessions. However, POD activities decreases in less tolerant accession with cold under treatment duration, whereas it is relatively maintained in tolerant accession. On the other hand, the data indicated that expression of isozymes in response to cold stress depends on the duration and is higher in tolerant than in sensible one.
Species of Medicago are interesting because of their adaptability to different soil and climates, their good winter growth, self-reseeding, they provide an input-saving and resource- conserving alternative because they fix atmospheric nitrogen, thus reducing the need for chemical fertilizers while enhancing overall crop productivity. In farming system, they are often used as an inter-crop (e.g., combined with cereals) or in crop rotation resulting in a decrease in pests, diseases and weed populations (Bullita et al., 1994; Araujo et al., 2015).
The production of a crop is challenged by abiotic and biotic stresses. Temperature is one of the most important environmental factors controlling seed germination, seedling heterotrophic growth, development of seedlings growth and development of the adult plant and also limiting crop distribution. During crop establishment, extreme temperatures can decrease plant emergence and lead to drastic losses in crop yield and quality (Dias et al., 2010; Kim and Tai, 2011; Avia et al., 2013). Up to 15 percent of the world’s agricultural production is lost to frost (Zou et al., 2007).
Metabolic activities in various cellular compartments, during normal cell metabolism, lead to the production of reactive oxygen species (ROS). These species mainly include superoxide radicals (O2−), singlet oxygen (1O2), hydrogen peroxide (H2O2), and hydroxyl radical (OH−). During abiotic and biotic stress conditions, the production of ROS increases to lethal levels. These highly reactive molecules can react with many cellular bimolecular and other components and damage DNA, proteins, and lipids. Thus, their concentration has to be tightly controlled. To counter the deleterious effects of ROS, aerobic organisms are equipped with antioxidant systems to scavenge ROS from the cells (Dasgupta et al., 2013; Teotia and Sing, 2014).
Reactive oxygen species (ROS) can be produced in plants exposed to low non-freezing temperatures. To minimize this oxidative damage, plants have evolved various enzymatic and non-enzymatic defense mechanisms to detoxify free radicals and reduce oxidative stress (Scebba et al., 1998; Mohammadian et al., 2012). Changes of peroxidase is generally related to stress responses (Sudha and Ravishankar, 2002; Syros et al., 2004; Zou et al., 2007). To prevent or alleviate cold oxidative injury, plants have evolved several mechanisms which include scavenging by antioxidant systems such as superoxide dismutase, catalase and peroxidase (Omran, 1980; Janda et al., 2003; Li et al., 2010; Moieni-Korbekandi et al., 2013; Fan et al., 2014).
In Triticum aestivum, seedlings exposed under cold acclimation showed higher peroxidase activity in frost resistant cultivar than in less frost resistant one. These changes in the activities of antioxidant enzymes induced by cold acclimation support the hypothesis that a frost-resistant wheat cultivar, in comparison with less frost-resistant one, maintains a better defense against ROS during low-temperature treatment (Scebba et al., 1998).
Kuk et al. (2003) showed, in rice plants, that isozyme profile and activity of peroxidase were significantly expressed under cold stress and deduced that peroxidase were most important for cold acclimation and chilling tolerance.
Two contrasting varieties, of rape seed (Brassica napus L.), for cold stress exhibit a distinct difference in the peroxidase. These changes were higher in cold tolerant varieties than in cold sensitive variety (Zou et al., 2007).
Liu et al. (2013) reported that POD activities in naked oats (Avena nuda L.) were higher under low temperature than normal temperature. POD activities is slowly increased in the first three days, rapidly increased in the third to fifth days, and reached the max in the fifth days, more than 4 times of control. The increased POD activities under low temperature improve cold tolerance in some degree. But with time POD activities decreased greatly, indicating that low temperature had affected POD enzyme. It may be due to low temperature affect on RNA transcription and translation, reducing the synthesis of POD. This authors show also, results imply that higher POD activities enhanced the capacity for scavenging ROS and contributed to enhanced tolerance of plant to cold stress.
Dai et al. (2009) reported that in two contrasting cold tolerance cultivars of barley, tolerant cultivar (M0103) had significantly higher peroxidase activity than the sensible cultivar (Chumai) after 72 h recovery in cold treated plants.
Fan et al. (2014) highlighted, in bermudagrass (C. dactylon) under cold stress, that peroxidase activities were higher in the cold regime than in the control, and the expression of antioxidant genes including MnSOD, Cu/ZnSOD, POD and APX was vividly up-regulated after cold stress.
The aims of this work was investigated changes of peroxidase antioxydative activity in annuals accessions of Medicago differing in cold tolerance collected from contrasting Algerian eco-geographic sites and try offer some referential criteria in terms of biochemical tools in Medicago annuals population with high tolerance, as well as their selection and breeding.
1. Results
1.1 Antioxidant peroxidase (POD) activity
Alteration of peroxidases activities was observed with cold stress, these induce an increase in the amount of quantitative and qualitative POD at the beginning of stress in both types, tolerant and less tolerant plants in all four species. But this activity decreases with duration of stress. However, this decrease was displayed in sensitive than in tolerant accessions.
The observed changes in peroxidase activity under cold treatment included significant differences between treatments (p<0.05) and accessions (p<0.05) based on ANOVA (data not shown). The mean accession peroxidases activity in comparison to control showed a significant increase after cold treatment at the different durations (Table 2 and 3). The results showed that under normal conditions, the peroxidase activity was greatest during the first days of seedling growth (2-6 days), then it is less solicited during the 8-10 days phase to be increased during the growth stage 11-14 days. Also, the results revealed that under cold regime peroxidase activity had different levels at the different durations of treatment. Peroxidase activities were higher under low temperature than normal in tolerant accessions than in sensible one. POD activities was slowly increased in first five days and increased in the 8 and 11 days under cold stress. This trend was more pronounced among tolerant accessions than in sensitive.
In M. polymorpha, after 5 days of cold regime, POD activity in tolerant (Poly 136) accession was 0.018 µmole against only 0.012 µmole in the sensible one (Poly 57). POD activity was maintained in comparison with untreated plantlets at T2 and T3 in tolerant accession than in the sensible one. Indeed, the results showed that the activity down 0.015 µ mole (T2) to 0.008 µ mole (T3) in Poly 57, While it was higher in T3 (0.013µ mole) than in T2 (0.011 µmole) in Poly 136 (Table 2).
|
|
|
Table 3 Mean ± σ of peroxidase (POD) (µ mol·min-1·g-1 FM) activity in M. aculeata (Ac 15679 and Ac 80) and M. truncatula (Tru 210 and Tru 26) |
The study was carried out in the plant tissue culture laboratory at the National Potato Research Centre, Tigon
Regarding peroxidase activity in M. ciliaris, cold treatment caused an increase POD activity in sensible (Cil 123) and tolerant (Cil 124) accessions after five days of low temperature treatment (0.018 µmole and 0.015 µmole) respectively in comparison with control. However, the peroxidase activity drop of 50% in cold-sensitive accession at T2 and T3 cold treatment. But in tolerant accessions the activity was maintained at a higher level in the term (Table 2).
When peroxidase activities under cold stress at different durations were compared with untreated plantlets, in M. truncatula accessions, it was seen that POD activities had the different behaviors in comparison with M. polymorpha and M. ciliaris species. Indeed, POD activities under cold treatment was not greater than control. Under cold treatment T1 cold-tolerant (Tru 210) and cold-sensible showed 0.010 µ mole and 0.008 µ mole respectively, versus 0.013 µ mole and 0.009 µ mole in the corresponding control. Nevertheless, this activity was greater with Tru 210 (0.013 and 0.007 µ mole) than Tru 26 (0.008 and 0.005 µ mole) (Table 2).
When plantlets were treated under cold regime, in M. aculeata accessions, POD activity in cold-sensible one (Ac 15679) decreased highly depending on the duration of cold treatment, for example this activity of treated plantlets fall by 75% in comparison with untreated plantlets after 11 days of cold treatment. On the contrary, in cold tolerant (Ac 80) POD activity increase under cold treatment in comparison with control during 5 and 8 days cold treatment and at least decrease with 50% only after 11 days of treatment (Table 2).
1.2 POD isozyme expression under cold stress
The results showed that there was changes in intensities and number of isozyme bands during applied cold stress.
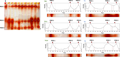
Analysis of peroxidases zymograms patterns, showed varied expression of isoforms in eight accessions studied, the number of these isozymes varies from 1 to 5 depending on each population studied. These isoforms, named POD 1-POD 5 were expressed in both tolerant and sensible accessions but variations in the intensity of the bands were observed among the days of cold treatment in comparison with the control (Figure 1A, and B to Figure 4A, and B).
|
|
|
Figure 4 Isoenzymes pattern of POD in M. polymorpha (A: Poly 136 and B: Poly 57) seedlings untreated (controls): lanes 1 to 8 (T02, T04, T06, T08, T010, T011, T012 and T014) and treated with different durations (T1, T2, and T3) under cold stress: lanes 9 to 11. The isoenzymes resolved are denoted by different numbers. Densitometric scans of POD isoenzymes on the profiles tolerant and sensible accession are showed in in the left of figure A and B respectively |
We noticed, in M. aculeata, five isozymes of POD (POD 1, POD 2, POD 3, POD 4 and POD 5) in the untreated control seedling in both tolerant and sensible accessions (Figure 1A, B). Some were expressed during the early stage of growth and disappeared at the later stages. In sensible accession (Ac 15679), POD 2 was shift at T1 and T2 treatment, but reappeared at T3 (Figure 1B).
When the plantlets were exposed 5 days to cold treatments (T1), in M. ciliaris, were observed four isozymes of POD (POD 1-POD 4) in the untreated control seedlings (Figure 2A, B). Particularly among the tolerant accession (Cil124), the expression of POD 2 was maintained after 8 and 11 days of treatment at low temperature while it was repressed in the less tolerant Cil 123. It would seem that only col-tolerant (Cil 124) has a capacity to activate POD 2 when stress is maintained over time.
|
|
In M. truncatula (Figure 3A, B), Tru 210 (cold-tolerant) presents the four major bands (POD 1-POD 4) from day 5 to 8 under cold regime and control, with high intensity under cold treatment for POD 3 and with less expression of POD 4. When the plantlets were exposed for 11 days at 4?, POD 4 is strongly expressed in comparison with control. In sensible accession (Tru 26), five POD appeared in untreated seedlings with variations depending on the development stage of plantlets. After a treatment at 4? for 5 and 8 days, POD 3 and POD 4 showed higher intensity compared to their respective controls. When the plantlets were exposed to cold stress for 11 days, POD 4 was slightly expressed and POD 5 was shift.
|
|
In M. polymorpha (Figure 4A, B), three to four isozymes of POD (POD 1, POD 2, POD 3, and POD 4) were observed in the untreated control seedling (Figure 4A, B). When the plantlets were exposed at 4?, tolerant accession expressed 4 isozymes whereas the sensible one expresses only 3 isozymes. Moreover, the intensity of POD expressed in tolerant were more important than in the sensible one. The relative levels of the mobility band were different among accessions tested. It appears in figure 4A, in tolerant accession (Poly 136), the isoenzyme POD 2 was activated under stress while it was not in normal condition. In contrast, in the sensitive accession (Poly 57) (Figure 4B), there was been no new isozyme activation. In addition, there has been a decrease in the activity (low intensity) at POD 1 isoenzyme when plantlets were exposed for 11 days under cold treatment.
2. Discussion and Conclusion
To our knowledge there is no available information relating to antioxidant activity of peroxidases and their expression under a low-temperature regime regarding natural populations of annual Medicago. Our study revealed that under normal conditions, the peroxidase activity is greatest during the first days of seedling growth (2-6 days), then it is less solicited during the 8-10 days phase to be increased during the growth stage 11-14 days. This has been highlighted by Moncousin and Ducreux (1984). This authors relating, by studying at the evolution of the activity of peroxidase, like scoring, during subsequent transplanting in Cynara scolymus L. that peroxidase activity varies during the induction phase and root initiation and the decrease of peroxidase activity is progressively with age of the plant. The work of Bogdanovic et al. (2008) argue in favor of correlation between POD isoenzymes and activity with early C. mural seedling development not involved in seed germination.
Whereas under cold stress, the peroxidase activities (POD) were gradually increased at the beginning of stress then decreased as the stress is maintained in the time. Moreover, this decrease is less marked among in tolerant accessions than in less tolerant ones. Zhang et al. (2008) suggested that, in the strawberry plantlets treated with 0?, a significant enhancement in the activities of peroxidase. In response to low temperature in two spring cultivars of canola (Brassica napus L.), Moieni-Korbekandi et al. (2013) revealed that there was a linear relationship between the increase of peroxidase activity with time in the second period of low temperature and the most increased POD activity under low temperature was on the fourth day in both sensible and tolerant cultivars.
Dai et al. (2009) reported, in sensitive and tolerant barley cultivars, that peroxidase activities was higher in cold-acclimated plants than in non-acclimated ones. This trend was observed also in chickpea (Cicer orientum L.) following cold acclimation, Nazari et al. (2012) reported that tolerant genotype showed higher activity when cold treatment is longer. Several cultivars of wheat exposed to 4?, showed a significant increases of peroxidase activities, this increase was more obvious in “CH Nortar winter” cultivar over the experimental period (Javadian et al., 2010). Moieni-Korbekandi et al. (2013) studied responses to low temperature in two spring cultivars of canola (Brassica napus L.), revealed that there was a linear relationship between the increase of peroxidase activity with time in the second period of low temperature and the most increased POD activity under low temperature was on the fourth day in both sensible and tolerant cultivars. Kang and Saltveit (2001) reported that cold stress induces the synthesis and accumulation of ROS that have been associated with injury development. Exposure to this stress induces both higher levels of antioxidant enzyme activity and stress tolerance. Plants under abiotic stress have evolved a defense system against oxidative stress by increasing the activity of ROS-scavening enzyme. ROS can be scavenged by peroxidase (POD).
Liu et al. (2013) reported, in Avena nuda L. plants submitted to cold stress, that peroxidase decreases greatly in later days of cold treatment. These authors reported that it may be due to low temperature affected RNA transcription and translation, reducing the synthesis of peroxidase. This decreasing was more pronounced in sensible accessions than in tolerant one. In our results, the data showed that PODs activities in sensible accessions decreased greatly in later days of cold treatments, indicating that low temperature had affected POD enzyme. In bermudagrass, Fan et al. (2014) highlighted that peroxidase activities were higher in the cold regime than in the control. Janda et al. (2003) showed that there is highest correlation between the enzyme activity (guaiacol peroxidase) and frost tolerance in hardened leaves of cereals.
Our results showed that POD activities were highly correlated and increase in the plantlets with cold regime. This increases was different among species and with cold treatment durations and this activities were greater in cold-tolerant than in cold-sensible accessions. Tsgun et al. (2006) highlighted that high peroxidase activities under cold stress can be a better strategy to tolerate freezing temperature. Moieni- Korbekandi et al. (2013) showed that there is a difference in peroxidase activity with time of treatment and the nature of sensitive or tolerant cultivars under low temperature regime. Scebba et al. (1998) argue for the relationship between the high peroxidase activities is intimately linked with the molecular mass peroxidase isoform.
The results of our work support the above consideration and according for these, for example, the increased POD activities under low temperature in M. polymorpha was linked with expression of isoform POD 2 only in tolerant accession Poly 136. It seems that the cold tolerant accessions display a better overall peroxidase activity. Isozymes expression under cold regime, these results showed clearly that inactive loci under the normal state are induced under a low temperature regime in cold-tolerant accession and less expressed or unexpressed in cold-sensible accessions.
It can be concluded from the changes observed in most of the antioxidant peroxidase investigated that they the increase in the overall activity of peroxidases may result either from a neosynthesis of the enzyme or the activation of inactive pre-existing enzymes following cold-acclimation. In the other words, it appears that chilling tolerance that is exhibited by tolerant accessions with isoenzyme expression is not entirely constitutive, and that at least of it is developed during exposure to chilling temperature. At least, it appears that chilling tolerance that is exhibited by tolerant accessions with high isoenzyme expression is not entirely constitutive, and that at least of it is developed during exposure to chilling temperature. The increase in the overall activity of peroxidases may result either from a neosynthesis of the enzyme or the activation of inactive pre-existing enzymes following cold-acclimation.
3. Material and Methods
3.1 Plant materials
Seeds of eight accessions of 4 Medicago species (M. aculeata, M. truncatula, M. ciliaris Krocker. and M. polymorpha L.) (Table 1) differing in cold tolerance (Yahia et al., 2014) were first disinfected by dipping in 70% (v/v) ethanol (1 min), rinsed three times in distilled water and placed on Petri dishes containing universal compost. Ten seeds per lot were incubated in dark at the ambient room temperature. 3 days after seedlings were divided on two lots. Cold acclimated one under (4±2) ? (photoperiod 16/8 hrs., 6700 Lux) at different duration 5, 8, and 11 days (T1, T2, and T3), and non-acclimated (control) (T02, T04, T06, T08, T010, T011, T012 and T014) remained at (23 ± 2) ? in the same conditions of photoperiod and duration. The experimental layout was a completely randomized block design with 3 replications. We measured the quantitative and qualitative activities of these enzymes under normal and cold stress. Levels of enzyme activity were examined for both tolerant and sensible accessions at different durations, 5, 8, and 11 days at a low temperature (4?) (T1, T2, and T3) after 3 days of germination and their controls T08, T011, and T014 respectively.
|
|
3.2 Peroxidases (POD) extraction and electro- phoresis native gel made
Enzyme extractions were carried out at 4?. Plant tissues were frozen in liquid nitrogen and ground with an ice-cold pestle and mortar, and then extracted in 10 mM Tris-KCl (pH 6.8) and 1 mM PMSF according Syros et al. (2004) modified. The homogenate was centrifuged at 14 000 trs/min for 30 min. The supernatant was collected and stored in small aliquots at liquid nitrogen for analysis. The isozymes of antioxidant enzymes were visualized by non-denaturing gradient (4~10%) polyacrylamide gel electrophoresis on vertical slab gel (Hoffer, USA). Equal amounts of proteins (15 µL) were loaded on to each lane. The gel electrophoresis experiment was repeated tree times. To determine the pattern of peroxidase isoforms gels were stained and visualized by immersing the gels in 100 mL 0.2 M acetate buffer pH 4.6 added with 1% o-dianizidine (Sigma), dissolved in 2 mL 95% ethanol, and 200 µL 3% H2O2 at room temperature until the brown color appeared. Scanned Isozymes profiles gels were analyzed by Software GELANALYZER (Istvan Lazar Hungary Copyright, 2010).
3.2 Quantitative peroxidase activities
Peroxidase activity (EC 1.11.1.7) was assayed in reaction solution (3 mL) containing 0.2 M sodium acetate buffer pH 4.6, 1% o-dianizidine, 30 % H2O2 (Mac Adam et al., (1992) modified. The reaction was started by adding 10 µL crude extract, and the enzyme activity is monitored for every 15 seconds for 3 minutes using a spectrophotometer (UK- JENWAY 7305) at 460 as o-dianizidine oxidation, with 3 replications. Enzyme activity was expressed as µmol. of o-dianizidine oxidation. min-1·g-1 of fresh matter.
3.3 Data analysis
The antioxidant enzymes activities are the mean values of tree independents replication (n=3). Data were analyzed by analyses of variance (ANOVA) with the software STATISTICA Version 10.0 (STATISTICA Inc., France).
Acknowledgments
This work was supported by Research PNR project entitled ″Biotechnologie des Rhizobiums et Amélioration des Plantes″ (code Project 61) affiliated to the Algerian Ministry of Higher Education and Scientific Research.
Reference
Avia K., Pilet-Nayel M.-L., Bahrman N., Baranger A., Delbreil B., Fontaine V., Hamon C., Hanocq E., Niarquin M., Sellier H., Vuylsteker C., Prosperi J.-M., and Lejeune-Hénaut I., 2013, Genetic variability and QTL mapping of freezing tolerance and related traits in Medicago truncatula, Theor. Appl. Genet.
DOI 10.1007/s00122-013-2140-7
http://dx.doi.org/10.1007/s00122-013-2140-7
Araujo S.S., Beebe S., Crespi M., Delbreil B., Gonzalez E.M., Gruber V., Lejeune-Henaut I., Link W., Monteros M.J., Prats E., Rao I., Vadez V., and Vas Patto M.C., 2015, Abiotic stress responses in Legumes: Strategies used to cope with environmental challenges, Critical Reviews in Plant Sciences, 34: 237-280
http://dx.doi.org/10.1080/07352689.2014.898450
Bogdanovi? J., Raditi? K., and Mitrovi? A., 2008, Changes in activities of antioxidant enzymes during Chenopodium murale seed germination, Biologia Plantarum 52 (2): 396-400
http://dx.doi.org/10.1007/s10535-008-0083-7
Bullita S., Floris R., Hayward M.D., Loi A., Porqueddu C., and Veronesi F., 1994, Morphological and biochemical variation in Sardinian populations of Medicago polymorpha L. suitable for Mediterranean conditions Euphytica 77: 263-268
http://dx.doi.org/10.1007/BF02262640
Dai F., Huang Y., Zhou M., and Zhang G., 2009, The influence of cold acclimation on antioxidative enzymes and antioxidants in sensitive and tolerant barley cultivars, Biologia Plantarum 53 (2): 257-262
http://dx.doi.org/10.1007/s10535-009-0048-5
Dias, B.M.P., Brunel-Muguet S., Dürr C., Huget T., Demilly D., Wagner M-H., and Teulat-Merah B., 2010, QTL analysis of seed germination and pre-emergence growth at extreme temperatures in Medicago truncatula, Theor. Appl. Genet. Published online. doi :10.1007/s00122-010-1458-7
Dasgupta N., Biswas P., Kumar R., Kumar N., Bera B;, and Das S., 2013, Antioxidant and ROS scavening ability in ten Darjeeling tea clones may serve as markers for selection of potentially adapted clones against abiotic stress, Physiol. Mol. Biol. Plants
DOI 10. 1007/s12298-013-0187-1
Fan J., Ren J., Zhu W., Amombo E., Fu J., and Chen L., 2014, Antioxidant responses and gene expression in Bermudagrass under cold stress, J. Amer. Soc. Hort. Sci. 139 (6): 699-705
Iba K., 2002, Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance, Annu. Rev. Plant Biol. 53: 225-245
http://dx.doi.org/10.1146/annurev.arplant.53.100201.160729 PMid:12221974
Janda T., Szalai G., Rios-Gonzalez K., Veisz O., and Paldi E., 2003, Comparative study of frost tolerance and antioxidant activity in cereals, Plant Science 164: 301-306
http://dx.doi.org/10.1016/S0168-9452(02)00414-4
Javadian N., Karimzadeh G., Mahfoozi S., and Ghanati F., 2010, Cold-induced changes of enzymes, proline, carbohydrates, and chlorophyll in wheat, Russian Journal of Plant Physiology 57 (4): 540-547
http://dx.doi.org/10.1134/S1021443710040126
Kang H.M., and Salveit M.E., 2001, Activity of enzymatic antioxidant defense systems in chilled and the heat shoced cucumber seedling radicles, Physiologia Plantarum pp: 548-556
http://dx.doi.org/10.1034/j.1399-3054.2001.1130414.x
Kim S.I., and Tai T.H., 2011, Evaluation of seedling cold tolerance in rice cultivars: a comparison of visual rating and quantitative indicators of physiological changes, Euphytica 178: 437-447
http://dx.doi.org/10.1007/s10681-010-0343-4
Kuk Y., In., S.J.S., Burgos N.R., Hwang T., Eak H.O., Cho B.H., Jung S., and Guh J.O., 2003, Antioxidative enzymes offer protection from chilling damage in rice plants, Crop Sci. 43: 2109-2117
http://dx.doi.org/10.2135/cropsci2003.2109
Li Jin-Ting., Qiu Zong-Bo., Xiao-Wei., and Wang Lin-Song., 2010, exogenous hydrogen peroxide can enhance tolerance of wheat seedlings to salt stress, Acta Physiol. Plant.
DOI 10.1007/s11738-010-0608-5
http://dx.doi.org/10.1007/s11738-010-0608-5
Liu W., Yu K., He T., Li F., Zhang D., and Liu J., 2013, The Low Temperature Induced Physiological Responses of Avena nuda L., a Cold-Tolerant Plant Species, The Scientific World Journal. doi.org/10.1155/2013/658793
Mac Adam J.W., Nelson C.J., and Sharpe R.E., 1992, Peroxidase activity in the leaf elongation zone of tall fescue, Plant Physiol. 99: 872-878
http://dx.doi.org/10.1104/pp.99.3.872
Mohammadian M.A., Largani Z.K., and Sajedi R.H., 2012, Quantitative and qualitative comparison of antioxidant activity in the flavedo tissue of three cultivars of citrus under cold stress, Australian Journal of Crop Science 6 (3): 402-406
Moieni-Korbekandi Z., Karimzadeh G., and Sharifi M., 2013, Ealuation of total soluble protein and antioxidant activities in two spring cultivars of canola (Brassica napus L.) in response to low temperature, International Journal of Agriculture and Crop Sciences 5 (4): 401-409
Moncousin C., and Ducreux G., 1984, Activité peroxydasique et rhizogenèse dans le cas Cynara scolymus L.: évolution au cours de repiquages successifs de bouture cultivées in vitro. Comparaison avec de jeunes plantes issues de graines, Agronomie 4(2): 105-111
http://dx.doi.org/10.1051/agro:19840201
Nazari M., Maali Amiri R., Mehraban F.Z., and Khaneghah H.Z., 2012, Change in antioxidant responses against oxidative damage in black chickpea following cold acclimation, Russian Journal of Plant Physiology 59 (2): 183-189
http://dx.doi.org/10.1134/S102144371201013X
Omran R.F., 1980, Peroxide levels and the activities of catalase, peroxidase, and indoleacetic acid oxidase during and after chilling cucumber seedlings, Plant Physiol. 65: 407-408
http://dx.doi.org/10.1104/pp.65.2.407
PMid:16661201 PMCid:PMC440338
Scebba Francesca., Sebastiani Luca., and Vitagliano Claudio., 1998, Changes in activity of antioxidative enzymes in wheat (Triticum aestivum) seedlings under cold acclimation, Physiologia Plantarum, 104: 747-752
http://dx.doi.org/10.1034/j.1399-3054.1998.1040433.x
Syros T., Yupsanis T., Zafiriadis H., and Economou A.? 2004, Activity and isoforms of peroxidases, lignin and anatomy during adventitious rooting in cuttings of Ebunus cretica L., J. Plant Physiol. 161: 69-77
http://dx.doi.org/10.1078/0176-1617-00938
PMid:15002666
Sudha G., and Ravishankar G.A., 2002, Involvement and interaction of various signaling compounds on the plant metabolic events during defense response, resistance to stress factors, formation of secondary metabolites and their molecular aspects, Plant Cell. Tissue and Organ Culture, 71: 181-212
http://dx.doi.org/10.1023/A:1020336626361
Teotia S., and Singh D., 2014, Oxidative stress in plants and its management. Approaches to Plants Stress and their Management, pp 227-253
Thind S.K., and Goyal P., 2012, Oxidative stress and antioxidant machinery of plants. LS An International Journal of Life Sciences.
Doi: 10.5958/j.2319-118x.1.2.009
http://dx.doi.org/10.5958/j.2319-118X.1.2.009
Tsgun E., Atici O., Nalbantoglu B., and Popova L.P., 2006, Effect of salicylic acid and cold treatment on protein levels and on the activities of antioxidant enzymes in the apoplaste of winter wheat leaves, Phytochemistry 67:710-715
http://dx.doi.org/10.1016/j.phytochem.2006.01.022
PMid:16519911
Yahia N., Fyad-Lameche F.Z., Bakhti N., and Barre P., 2014, Characterization of Medicago populations under cold acclimation by morphological traits and microsatellites (SSR) markers, African Journal of Biotechnology, 13(27): 2704-2714
http://dx.doi.org/10.5897/AJB2014.13788
Zhang Y., Tang H.R., and Luo Y., 2008, Variation in antioxidant enzyme activities of two straw berry cultivars with short-term low temperature stress, World Journal of Agricultural Sciences 4 (4): 458-462
Zou W.H., Chen Y.Z., and Lu C.F., 2007, Differences in biochemical responses to cold stress in two contrasting varieties of rape seed (Brassica napus L.). For. Stud. China. 9 (2): 142
http://dx.doi.org/10.1007/s11632-007-0022-2
. PDF(4405KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Yahia Nourredine
. Ali Naima
. Habouche Dalila
. Semache Habib
. Saad Karim
. Fyad-Lamèch Zohra
Related articles
. Cold stress
. Annual accessions
. Medicago
. Peroxidase (POD) (EC 1.11.1.7)
. Antioxidant activities
Tools
. Email to a friend
. Post a comment
.png)
.png)
 xiao.png)

.png)
.png)
.png)

.png)


