 Author
Author  Correspondence author
Correspondence author
Molecular Plant Breeding, 2015, Vol. 6, No. 14 doi: 10.5376/mpb.2015.06.0014
Received: 22 Jun., 2015 Accepted: 11 Aug., 2015 Published: 08 Oct., 2015
Das K. and Adak M.K., 2015, Biosorption Property of Cadmium (II) in Marsilea minuta L. with Polyamine Interaction, Molecular Plant Breeding, 6(14): 1-4 (doi: 10.5376/mpb.2015.06.0014)
A study was undertaken for biosorption following bio accumulation property of Marsilea minuta L., an aquatic pteridophyte in cadmium enriched solutions. The possible mechanism for biosorption on the cell wall residues has been discussed with exogenous applications of polyamine. Initially plants were evaluated both with fresh and dry mass for their differential adsorption. A typical distribution of metal in different tissues, more into cortical and endodermal apoplastic regions were the features as detected by Energy Dispersive Analysis of X-ray study. The changes of the cell wall residues and their possible interaction with metals have been analyzed with Fourier Transform Infrared Spectrometric studies. With this study the most possible contributing chemical bond was detected as alkyl halide with its possible changes in energy absorption. This was also minimized by spermidine application and thus established its involvement for biosorption of metal. Accumulation of Cd in relation to chemical mechanism has been discussed in Marsilea and analyzed its efficacy as a well hyper-accumulator under metal contaminated environment.
Introduction
The toxicity for heavy metal has undoubtedly been a subject of serious concern with regards to plant’s nutrition. Accumulation of the heavy metals in different degrees and duration poses a hazard to realize the growth and development of vegetation. Of the most discussed toxic and heavy metals in plant system, the cadmium (Cd) might be paid the highest attention in damages of plant growth and subsequent hazards for animals and human (Pielichowska and Wierzbicka, 2004) Along with the rapid and enormous uptake of Cd, it becomes more detrimental to plant’s at the level of cellular and physiological processes (Das et al., 2013) Likewise, starting from seed germination, Cd directly or indirectly interferes many life processes including carbon assimilation (Lemoine et al., 2013), its allocation, energy yielding metabolism, impaired water relation, evocation of oxidative stress and finally inadequate biomass and low economic yield (Ludewig and Flugge, 2013). At the cellular level, Cd is sensitized as a pro-oxidant with generation of various reactive oxygen intermediates/species (ROI/ROS) for establishment of oxidative stress (Benavides et al., 2005). A number of bio-molecules both at cytosolic and sub-cellular fractions are undergo oxidative damages and thereby curtailing the metabolic pathways. In addition to cytosolic or cellular paths, plants, however, are preliminarily affected by interaction of the Cd on its non-cellular path. Those include the cell wall, vacuoles and other apoplstic spaces in a significant amount (Tariq et al., 2014). This is technically referred as biosorption which is prelude to bio-accumulation process of Cd or other heavy metals. With the case of removal of the metals, the biosorption is referred as reversible and non-energy driven surface binding event. This biosorption of heavy metal has been a very efficient and promising phenomenon involving the removal of the pollutants mostly the xenobiotic and derivatives with heavy metal residues. This is more accomplished from contaminated bodies especially from waste water either of natural or industrial in sources (Vassilev et al., 2002).
According to plant species a significant variations have been found for the degrees of sensitivity to Cd. This gives the advantages for probing the hyper accumulating plant species for their ability in phytoextraction of metals from soil at a higher rate. A number of plant species has been paid attention for their genetic advantages of hyper accumulation of metals (Singh et al., 2010). It is quite in agreement that plant’s responses to heavy metal stress very often undergo interacted by some chemical elicitors either endogenous or exogenous in application (Hossain et al., 2012). The elicitors have been played to modulate the metal absorption in a number of ways including adsorption of the ion on the cell wall (facilitated diffusion of the ions over the membrane, moderation of metal induction for ROS development and finally transduce the signal into nucleus for gene expression. The later is manifested in amelioration of the metal induced damages (Gill and Tuteja 2010). However, the actual insight for the role of apoplast in metal transport and its immobilization within the tissues are less studied, particularly, in lower groups of plants. Admitted well that cell wall matrix and its projected chemical moieties have been intrinsic affinity for adsorption of different metallic ions. This very often undergoes some deviations with metal hindrance with reference to bending and stretching of bonds furnished byfunctional groups (Vasconcelos et al., 2014). Those include complex polysaccharides, proteins, lipid with glycerophosphate residues etc. It is also reported that those chemicals are often shown to be easily biodegradable, forming chemical conjugants with the metals on the cell wall or even within the apoplastic spaces (Nguema-Ona et al., 2014). Thus, hyper-accu- mulating plant species are escaped from the vulnerability of metal toxicity through inhibition of downstream paths into cytosol crossing the cell membrane by sorption of metals.
Amongst the elicitors found in plant system, polyamines have been implicated the most authentic one with regards to their nature and functionales. These cover their ubiquitous existence in tissues, low molecular weight, high solubility and diffusivity, non-hindering the cellular pH as well as with a wider span of physiological activities (Minocha et al., 2014). Polyamines have been encountered in a diverse mode of action for various abiotic stresses including metal toxicity, mostly in higher plants. Lower groups of plants representing the pteridophytic species have also been evaluated for polyamine sensitivity with special reference to metal stress also (Mandal et al., 2013). According to the molecular configuration of polyamines, the lower molecular weight moieties are most effectively used for their rapid diffusivity over the membrane as well as dose-dependent activity. Thus, putrescine (diamine), spermidine (triamine) and spermine (tetraamine) are very often used in plant responses to abiotic stresses (Hossain et al., 2012). Therefore, it could be an assumption that polyamines may have some influences in effects on the biosorption phenomenon of metals in plant species. Wide arrays of information have enriched the effectiveness of polyamines for biosorption of metal in angiospermic plants. However, for non-angiospermic plants the responses to polyamines are rather scares and essentially needs to explore its fate of application for metal remediation in soil. Despite this, some aquatic pteridophytes like Salvinia, Azolla which represents their tolerance to some specific heavy metals has set examples with reference to polyamine sensitivity (Mandal et al., 2013). Therefore, we hypothesize that some specific features enabling the metal resistance in fern species might be through adsorption phenomenon by polyamine moieties also. Thus, the present experiment is based on the evaluation of Marsilea plant selecting Cd as a heavy metal with interaction of spermidine for hyper-accum- ulation property. The changes of specific cell wall constituents with the interference of Cd toxicity in this species have been described in some details.
Result and Discussion
From the facts and figures of the present experiment with Marsilea plant incubated under different Cd concentrations, it recorded that plants were significantly affected with Cd bio-adsorption and accumulation. In our earlier studies, the bio-accumulation of Cd was found in parallel with concomitant concentrations of metal supplied (Sabeen et al., 2013). In addition to this, it had also documented with a significant changes of cell surfaces with some irregular patches under Cd induction (Das et al., 2014). In the present experiment, a detailed analysis and its possible interpretation was attempted with EDAX studies in Marsilea plants for Cd bio- adsorption (Figure 1a-1e). Removal of metals by biological remediation is essentially based on bio-adsorption followed by accumulation. The mechanism of this process involves precipitation of the metals in extracellular spaces and on cell surfaces (Gaur et al., 2014). This also may be hypothecated that cell surfaces might be furnishing with some functional groups projected from biomolecules of cell wall to anchor the adhering metals (Simon AA, García-Angulo P, Melida H, Encina A, Alvarez JM, Acebes JL 2011). In a mixture with more than one substance, particularly, with the biomolecules, there are some physical bending of functional groups against chemical interactions. This is more supported with changes in bio-adsorption pattern with varying states of biomass. In the present experiment also Marsilea plant had followed the same pattern. The dry and fresh biomass showed a significant variation in metal adsorption in a range of 5.15, 6.98, 13.35 fold and 1.97, 2.31, 4.2 fold respectively over control. With the application of spermidine (Spd), the plants were less absorbed by 67.48% and 48.97% in dry and fresh biomass respectively when compared to 200 µM Cd (Figure 2). This is interesting to note the changes in characteristic features of bands in FTIR analysis for different bio-molecules as cell wall constituting residues (Figure 3). In the present experiment, the spectra from varying Cd concentrations had shown appreciable variations. However, the peak maximum of each frequency was less altered but with some changes in the shapes as well as intensities also. FTIR analysis provides the spectra of each such mixing moiety with some characteristic peaks for chemical modifications. Likewise, the chemical changes of cell surfaces of Marsilea under Cd stress supplemented with Spd were featured by FTIR spectroscopy (Lesmana et al., 2009). The physicochemical analysis of this process suggested that bio-adsorption is facilitated by complication, coordination, ion-exchange, chelation and adsorption of metals. With reference to plants, the removal of metals is facilitated with an initial reversible bio-adsorption and secondarily, a slow but steady irreversible bio-accumulation. The latter is also referred to as ion sequestration when specific sub-cellular fractions are engaged in the tissues. This was more confirmed with FTIR studies that had shown in the figures (Figure 3). For the comparative analysis of samples from varying Cd concentrations and with Spd treatment, it recorded a distinct variable trend suggesting the impact of heavy metals on Marsilea and its responses on cell wall moieties. This was also confirmed and concluded from many experiments for the efficacy of plant biomass for bio-adsorption of metals. This was also documented from our Marsilea plant irrespective of dry and fresh state of biomass. From the tables of changes in the frequency regions of different functional groups of biomolecules on the cell wall are expected to be involved for heavy metal (e.g. Cd in the present case) adsorption (Table 1). This probably is indicative of the facts of heavy metal induced bio-adsorption with some functional groups with changes in bond strength. From the Table 1, the major affected functional groups are detected as alkyl halide (-C-Cl) with frequency region 770-772 cm-1, the amino functional group is more dissected with C=O (with frequency region 1652 cm-1 and N-H (with frequency region 3409-3399 cm-1) (Esteves et al., 2013). Moreover, in earlier studies, it reported that any changes in the fingerprint region of 1600-3400 cm-1 (amide –I and II) are due to excess amount of organic acids and non-proteinaceous or carbohydrate residues under exposure of Cd , Cu, Pb, Cr and Zn (Bartosova et al., 2013). Therefore, Cd being a heavy metal was bio-adsorbed on the cell surfaces with the contribution of a few functional groups like amide, hydroxyl, amine etc. and that also evident in the present experiment with Marsilea. Again, a linear decrease in frequency (772.21-770.64 cm-1) was observed from control biomass up to 200 µM of Cd treatment. More so, an increase in absorption frequency (772.21 cm-1) was noted in the biomass supplemented with Spd (Figure 3). This arises from the possible the interactive phenomenon of carboxyl group (–COOH) with Cd. This is well in conformity that presence of –COOH from different nucleophilic species those could furnish the bonding strength with divalent cations (Cd could be of those). However, Marsilea plant, being a typical water fern in nature has also registered the similar kind of metal induced damages mainly by direct loss of potassium ion (K+) etc along with some modification in cell wall components. Again Spd treatment has been evident to encounter in such a way that more possibly the alkyl halide (-C-Cl) groups are less stretched with moderate bending. So, alkyl halide evokes the possibility of most sensitive chemical residue furnishing the bio-adsorption of Cd in Marsilea. The major changes for three principal bio-molecules like carbohydrate, protein and lipid of their absorption variation at different wave length were given in Table 2. This may also be resumed that physical and chemical conformity of the cell wall and its plasticity could be the minimum requirement for hyper-accumulating property of any plant species at least initially. In fact, the plant species remains in some sort of disadvantageous condition by losing out essential elements like K+. This was confirmed with a typical EDAX spectrum in comparison with varying Cd concentrations against control (Figure 1a-1e). Thus, in the present experiment, Marsilea observed a depletion of K+ by 67.34 %, 94.94% and 95.58% in 50, 100 and 200 µM Cd concentration respectively as compared to controlled condition (Figure 1a-1e). However, the plants had recovered the K+ content by 6.6 % with Spd application. This could be related for the interaction of some transporter/channel proteins for ion binding as evident from other studies dealt with polyamines in relation to metal scavenging activities (Minocha et al., 2014). When maize roots were allowed to adsorb Pb ion under biodegradable chelating compounds, a significant comeback or recovery of other essential ions was observed (Fernando et al., 2007).Therefore, those biodegradable compounds behaved a good source of chemical elicitors for mitigation of heavy metal bio-accumulation. In the present case, Marsilea plants were also well responded to spermidine as elicitors to reduce the metal adsorption as well as loss of K+ in result.
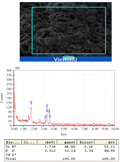 Figure 1 a-1e EDAX spectra of Marsilea plant of a) 0 µM Cd, b) 50 µM Cd, c) 100 µM Cd , d) 200 µM Cd and e) 200 µM Cd+2 mM spermidine |
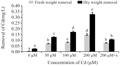 Figure 2 Adsorption of metals by Marsilea plant in fresh and dry sample states under different Cd concentrations |
 Figure 3 FTIR spectra of Marsilea plant under -0 µM Cd, 50 µM Cd, 100 µM Cd, 200 µM Cd and 200 µM Cd+2 mM spermidine |
|
Table 2 FTIR detected changes in absorption bands of protein, lipid and carbohydrate under Cd induction |
In conclusion, it is very clear that metal gets bio-adsorbed following interaction strongly with some functional groups. Thus, modification of cell wall takes place in such a way that undergoes irreversible damages. Marsilea plant being exclusively aquatic in nature have by now been reported a noticeable amount of heavy metal quenching ability (Lemoine et al., 2013). Therefore, the results of the present investigation may speculate that plants reacting with heavy metals or toxic ions are preliminary accomplished by the modification of cell wall, ion sequestering, binding with organic acids and peptide groups etc. This suggests a possible way for alleviation or mitigation of Cd toxicity, however, mostly offered in hyper-accu- mulating plant species. Thereby a fair chance for Cd removal in Marsilea plants do also exist as like other related aquatic plant species (e.g. water hyacinth) from organometallic pollutants in water bodies (Murithi et al., 2014). Taking all together, the changes of functional groups in chemical residues could be hypothesized for possible modification of cell wall to Cd toxicity. Thereby Marsilea plant could be assumed as an effective biomass for phytoremediation purposes. In addition, FTIR analysis coupled with EDAX study become evident as a potential tool for bio-adsorption analysis in plant species even in non-angiospermic plants like that of Marsilea minuta (L).
Materials and methods
Experimental treatments
Marsilea minuta L., was collected from ponds of University of Kalyani, Nadia having different micro and macro vegetations in the monsoon season. The plants were acclimatized in normal water for 7 days under ambient condition. For the experiments of EDAX and FTIR, Marsilea plants were grown in nutrient solution with one fourth strength of MS (Murashige and Skoog) media for 7 days supplemented with various concentrations (0, 50, 100 and 200 µM) of cadmium chloride (CdCl2) in different sets with at least four replications (Murashige and Skoog 1962) Another set was done with 2 mM spermidine (Spd) with 200 µM of CdCl2 solution. All the sets were incubated for 7 days in growth chamber with temperature of 37±1°C, 85% relative humidity and 14 h light (irradiance 72-80 µM/m2/s) and 10 h in dark. After completion of the incubation period, SEM, EDAX and FTIR experiments were done following the processes mentioned below. Statistical analysis was done with three replication per treatment on plants and was computed adopting the conventional ANOVA analysis.
Biosorption studies
For Biosorption, two types of plant materials like fresh and oven dried powder were taken in equal amount (2.0 g). For dried material, the plants were kept in hot air oven (80-90ºC) for 7 days till the constant weight comes. Each type of sample of equal weight was completely immersed in different Cd solutions (0, 50, 100 and 200 µM of CdCl2) for 24 h with constant agitation at 37°C. After incubation period was over, the plant samples were filtered through Whatman 42 filter paper and the filtrates were analyzed for Cd content through Atomic Absorption Spectrophotometry (Spectra AAS 240 Agilent Technologies, γ for Cd absorption-228.8 nm) .The amount of metal bioadsorbed by the leaf sample was calculated from the difference of Cd content before and after immersion of plant material in respective solutions (Pandey and Banerjee 2011).
All observations were recorded considering four replications (n = 4).The statistical analysis was performed by one-way ANOVA analysis taking P ≤ 0.05. The data presented in the figures are as mean value ±SE.
Detection of metal distribution in tissues.
Scanning Electron Microscopy (SEM) coupled with Energy Dispersive Analysis for X-ray (EDAX) was employed to monitor the cell surface variations with different concentrations of Cd adsorption (Srivastava and Thakur 2006). The distribution of metals in different proportions of the cells and Cd binding sites were detected by EDAX spectra of the biosorbent from surface pictures of SEM (JSM 6700 F, Japan)
Detection of functional groups for biosorption
The different functional groups present in the cell wall constituting moieties were done by Fourier Transform Infrared Spectrometry (FTIR). For FTIR analysis plant material was ground into fine powder and mixed with Potassium Bromide (KBr, AR) in 1:1000 p/p. The range of absorption spectra was between 400-4000 cm-1. In our experiment, three specific wavelengths for probable changes within the major biomolecules as carbohydrate (1200 cm-1-1000 cm-1), lipid substances (3000 cm-1 to-2800 cm-1) and protein (1800- cm-1 to 1500 cm-1) were considered. From the spectra obtained the possible changes of IR absorption was detected for specific functional groups as suggested (Luo et al., 2010)
Acknowledgement
The corresponding author acknowledges the partial financial support both from DST-PURSE programme activated to University of Kalyani. The K. Das, Assistant Prof. in Botany, acknowledges the FDP programme, UGC XIth Plan.
References
Bartosova A., Soldan M., Sirotiak M., Blinova L., and Michalikova A., 2013, Application of FTIR spectroscopy for determination of glucose in hydrolysates of selected starches, RESEARCH PAPERS Faculty of materials science and technology in trnava Slovak University of Technology In Bratislava Special Number, pp.116-121
Benavides M.P., Gallego S.M., and Tomaro M.L., 2005, Cadmium toxicity in plants, Brazilian Journal of Plant Physiology, 17: 21-344
http://dx.doi.org/10.1590/S1677-04202005000100003
Das K., Mandal C., Ghosh N., Dey N., and Adak M.K., 2013, Cadmium accumulation in Marsilea minuta Linn. and its antioxidative responses, American Journal of Plant Science, 4: 365-371
http://dx.doi.org/10.4236/ajps.2013.42A048
Das K., Mandal C., Ghosh N., Dey N., and Adak M.K., 2014, Responses of Marsilea minuta L. to cadmium stress and assessment of some oxidative biomarkers, American Journal of Plant Science, 5: 1467-1476
http://dx.doi.org/10.4236/ajps.2014.510162
Esteves B., Marques A.V., Domingos I., and Pereira H., 2013, Chemical changes of heat treated Pine and Eucalypt wood monitored by FTIR Maderas, Cienciay tecnología, 15: 245-258
Fernando B., Pereira F., de Abreu C.A., Romeiro S., Maria A, Lagoa M.A., and Gonzalez A.P., 2007, Pb-phytoextraction by maize in a Pb-EDTA treated oxisol, Scientia Agriculture, 64: 52-60
http://dx.doi.org/10.1590/S0103-90162007000100008
Gaur N., Flora G., Yadav M., and Tiwari A., 2014, A review with recent advancements on bioremediation-based abolition of heavy metals, Environmental Science Processes Impacts, 16: 180-193
http://dx.doi.org/10.1039/C3EM00491K
Gill S.S., and Tuteja N., 2010, Polyamines and abiotic stress tolerance in plants, Plant Signaling and Behaviour, 5: 26-33
http://dx.doi.org/10.4161/psb.5.1.10291
Hossain M.A., Piyatida P., daSilva J.A.T., and Fujita M., 2012, Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation, Journal of Botany, 2012: 1-37
http://dx.doi.org/10.1155/2012/872875
Lemoine R., Camera S.L., Atanassova R., Dedaldechamp F., AllarioT., Bonnemain J.L., Laloi M., Thevenot P.C., Pourtau N., Maurousset L., Faucher M., Girousse C., Lemonnier P., Parrilla J., and Durand M., 2013, Source sink transport of sugar and regulation by environmental factors, Frontier in Plant Sciences, 4: 1-21
http://dx.doi.org/10.3389/fpls.2013.00272
Lesmana S.O., Febriana N., Soetaredjo F.E., Sunarso J., and Ismadji S., 2009, Studies on potential applications of biomass for the separation of heavy metals from water and waste water, Biochemical Engineering Journal, 144: 19-41
http://dx.doi.org/10.1016/j.bej.2008.12.009
Ludewig F., and Flugge U., 2013, Role of metabolite transporters in source sink carbon allocation, Frontier in Plant Sciences, 4: 1-16
http://dx.doi.org/10.3389/fpls.2013.00231
Luo J., Xiao X., and Luo S.L., 2010, Biosorption of cadmium (II) from aqueous solutions by industrial fungus Rhizopus cohnii, Translocation of nonferrous, Metals Society of China, 20: 1104-1111
Mandal C., Ghosh N., Dey N., and Adak M.K., 2013, Physiological responses of Salvinia natans L. to aluminium stress and Its interaction with putrescine, Journal of Stress Physiology and Biochemistry, 9: 163-179
Mandal C., Ghosh N., Maiti S., Das K., Gupta S., Dey N., and Adak M.K., 2013, Antioxidative responses of Salvinia (Salvinia natans L.) to aluminium stress and it’s modulation by polyamine, Physiology and Molecular Biology of Plants, 9: 91-103
http://dx.doi.org/10.1007/s12298-012-0144-4
Minocha R., Majumdar R., and Minocha S.C., 2014, Polyamines and abiotic stress in plants: a complex relationship, Frontier in Plant Science, 5: 1-37
http://dx.doi.org/10.3389/fpls.2014.00175
Murashige T., and Skoog F., 1962, A revised medium for rapid growth and bioassays with tobacco tissue cultures, Physiologia Plantarum, 15: 473-497
http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x
Murithi G., Onindo C., Wambu E., and Muthakia G., 2014, Removal of cadmium to ions from water by adsorption using water hyacinth (Eichhornia crassipes L.) biomass, Bio. Resources, 9: 3613-3631
http://dx.doi.org/10.15376/biores.9.2.3613-3631
Nguema-Ona E., Vicre-Gibouin M., Gotte M., Plancot B., Lerouge P., Bardor M., and Driouich A., 2014, Cell wall O-glycoproteins and N-glycoproteins: aspects of biosynthesis and function, Frontier in Plant Sciences, 5: 499
http://dx.doi.org/10.3389/fpls.2014.00499
Pandey A., and Banerjee D., 2011, Biosprption of cadmium (II) using discarded biomass of Aspergillus aculeatus DBF9, Terrestrial and Aquatic Environmental Toxocology, 6: 8-13
Pielichowska M., and Wierzbicka M., 2004, Uptake and localization of cadmium by Biscutella laevigata, a cadmium hyperaccumulator, Acta Biologica Cracoviensia Series Botanica, 46: 57-63
Sabeen M., Mahmood Q., Muhammad I., Iftikhar F., Khan A., Farid U., Hussain J., Yousaf H., and Tabassum S., 2013, Cadmium phytoremediation by Arundo donax L. from contaminated soil and water, Bio. Medical Research International, 2013: 1-9
http://dx.doi.org/10.1155/2013/324830
Simon A.A., García-Angulo P., Melida H., Encina A., Alvarez J.M., and Acebes J.L., 2011, The use of FTIR spectroscopy to monitor modifications in plant cell wall architecture caused by cellulose biosynthesis inhibitors, Plant Signaling and Behaviour, 6: 1104-1110
http://dx.doi.org/10.4161/psb.6.8.15793
Singh R., Singh D.P., Kumar N., Bhargava S.K., and Barman S.C., 2010, Accumulation and translocation of heavy metals in soil and plants from fly ash contaminated area, Journal of Environmental Biology, 31: 421-430
Srivastava S., and Thakur I.S., 2006, Biosorption potency of Aspergillus niger for removal of chromium (IV), Current Microbiology, 53: 232-237
http://dx.doi.org/10.1007/s00284-006-0103-9
Tariq J.M., Lindberg S., and Greger M., 2014, Cellular proton dynamics in Elodea canadensis leaves induced by cadmium, Plant Physiology and Biochemistry, 77: 15-22
http://dx.doi.org/10.1016/j.plaphy.2014.01.009
Vasconcelos M.W., 2014, Chitosan and chitooligosaccharide utilization in phytoremediation and biofortification programs: current knowledge and future perspectives, Frontier in Plant Science, 5: 1-3
http://dx.doi.org/10.3389/fpls.2014.00616
Vassilev A., Vangronsveld J., and Yordanov I., 2002, Cadmium phytoextraction: present state, biological backgrounds and research needs, Bulgarian Journal of Plant Physiology, 28: 68-95
. PDF(797KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Das K.
. Adak M.K.
Related articles
. Biosorption
. Polyamine
. Marsilea minuta
. FTIR
Tools
. Email to a friend
. Post a comment


