Cloning of SCA Gene Related to Pollen Tube Adhesion and Oriented Growth and Analysis of Gene Diversity in Lilium spp. 
 Author
Author  Correspondence author
Correspondence author
Molecular Plant Breeding, 2011, Vol. 2, No. 16 doi: 10.5376/mpb.2011.02.0016
Received: 30 Oct., 2011 Accepted: 07 Nov., 2011 Published: 18 Jan., 2012
Wu et al., 2011, Cloning of SCA Gene Related to Pollen Tube Adhesion and Oriented Growth and Analysis of Gene Diversity in Lilium spp., Molecular Plant Breeding, Vol.2, No.16 109-118 (doi: 10.5376/mpb.2011.02.0016)
Stigma/style cysteine-rich adhesin genes (SCA) were cloned from ‘Tresor’ (Lilium Asiatic Hybrids), ‘White heaven’ (Lilium Longiflorum Hybrids) and ‘Caruso’ (Lilium Oriental Hybrids) leaves by PCR approaches. In this research we obtained four gene copies in both of ‘Tresor’ and ‘Caruse’ genomes and three gene copies in ‘White heaven’ genome. The identities of the cloned SCA among the copies were in the ranges from 72.97% to 99.68%. There are two exons and one intron in all of cloned SCA sequence, which could be deduced immature protein with 113 or 115 amino acid residues (encoding mature protein with 91 amino acid residues) SCA protein contains an N-terminal signal peptide of typical LTP protein, eight conserved cysteine residues and two consensus pentapeptide motifs. The identities among the amino acid sequences were in range from 87.91% to 98.90% higher than that of the similarities of gene sequences. Alignment analysis of amino acid sequences of cloned SCAs showed there were some distinct differences of amino acid site existing in the tested three species of lily hybrids. The phylogenetic tree revealed the relationship of the cloned 11 amino acid sequences of SCA were all more close to LTP family of monocotyledons than that of dicotyledons, which were consistent with the conclusions of kinship in plant taxonomy.
In the process of affinity pollination, the paternal pollen germinates on the papillate cells of maternal stigma, pollen tube goes into the style through the papillate cells, directional growing from the top in the style into the ovary, and then going into placenta and micropyle from the ovary, of which pollen tube growth precise and orderly exhibits a serial of directional growth that would be related with the cascade regulations of genes in different growth stages (Higashiyama, 2010). The adhesion and directed growth of pollen tubes in the style would be an important link to affect whether the pollen tube could go through the style of stigma successfully, as well as to affect directional growth of pollen tube toward the micropylar cap in the later growth (Palanivelu and Preuss, 2006). Therefore, it would be helpful to partly explain the molecular mechanism of hybridizing barriers among the lily species by isolating the genes (SCA genes) related to pollen tube adhesion and directional growth of lily, identifying gene functions and studying the influences of the SCA gene on pollen tube adhesion and directed growth in the same species or different species.
SCA (Stigma / style Cysteine-rich Adhesin) protein was isolated from stigma and style secretions of Easter lily (Lilium longiflorum Hybrids) 'Nellie white' reported by Lord’s laboratory of the University of California, of which SCA protein could promote pollen tube adhering to epidermis of transmitting tissue of the style with the assistance of pectin polysaccharides as well as promote mutual adhesion of pollen tubes (Mollet et al., 2000, Park et al., 2000). Lord’s laboratory also had isolated a Chemocyanin protein that promotes pollen tube directional growth from the stigma towards the style, while SCA protein could enhance the function of such directional growth (Kim et al., 2003).
At present, lily commercial varieties mainly belong to three hybrid species, that is Asian lily (Lilium Asian hybrids), Oriental lily (Lilium Oriental hybrids) and Easter lily (Lilium Longiflorum hybrids). It is easier to succeed in hybrid breeding of intra-species but more difficult to affinity of interspecies (van Tuyl et al., 2000). However, hybrid of interspecies, such as OA hybrid species, LA hybrid species and LO hybrid species, usually has a much more ornamental value or higher stress resistance, being worthy to be developed. Regarding interspecies crossing, although in vitro integrated technology was invented (integration of in vitro pollination, in vitro ovary culture, in vitro ovule culture and in vitro embryo rescue technology) (van Tuyl et al., 1991), the hybrid success rate is still quite low (van Tuyl et al., 2000) because the kinship of the parents is too far leading to the directional growth ability of paternal pollen tube in the maternal pistil is greatly reduced, of which the pollen tube in upper part of the style cannot occur directionally through lower part of the style to the ovary, or the pollen tube in the ovary cannot turn directionally toward the micropyle. The functional studies of lily SCA genes promoting the pollen tube adhesion and directional growth in Lord’s laboratory attracted our research interesting, it might be able to provide some helps to feature out the mechanism of hybridizing barriers among lily species.
In order to feature out SCA gene structure and explore the differences of the genes among the different hybrid lines of lily, we chose one of the varieties from Asian lily, Oriental lily, and Easter lily, respectively, to isolate SCA genes as well as to analyze the gene functions in this study, which might provide some research basis to explain the mechanism of hybridizing barriers.
1 Results and Analysis
1.1 Specific PCR amplification for Lily SCA
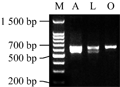
PCR amplification was performed with the primer of LSCAF / LSCAR in three different varieties of lily genomic DNA to generate the fragments with the ranges from 500 to 700 bp in size (Figure 1). The PCR products were cloned and sequenced. In this research we obtained four different sequences from the Asian Lily 'Tresor' and Oriental Lily 'Caruso', respectively, as well as three different sequences from Easter lily 'White heaven'. Four sequences of the Asian lily were named as LaSCA1, LaSCA2, LaSCA3 and LaSCA4 as well as the four sequences of Oriental lily named LoSCA1, LoSCA2, LoSCA3 and LoSCA4, while the three sequences of Easter lily was named LlSCA1, LlSCA2 and LlSCA3.
|
|
1.2 DNA sequence analysis of lily SCA
In this research, the full length genes were amplified by a pair of primer. The sequences compared with SCA cDNA sequence of Easter lily 'Nellie white' exhibited that the amplified genes containing two exons and one intron (Table 1). The intron splicing site was in line with the classic GT-AG rule (Figure 2). In previous study, the LTP gene containing intron was assigned to type â… , while the gene without intron was assigned to type â…¡ (Yubero-Serrano et al., 2003). The obtained SCA genes would be type â… gene, of which presented great variations in the intron but quite conservation in the exons of the coding region (Figure 2; Table 1). Eleven SCA gene sequences were deposited in the GenBank with the corresponding accession number (Table 1). Similarity of 11 sequences was performed by using DNAMAN to exhibit sequence similarity ranged from 72.97% to 99.68% (Table 2).
|
|
|
|
|
|
1.3 Comparison of Lily SCA similarity and amino acid sequence analysis
Amino acid sequences of mature protein corresponding the cloned sequences were deduced based on determination of slicing signal of intron, open reading frame and signal peptide. The amino acid sequence similarity was in the range from 87.91% to 98.90% for higher than that of the nucleotide sequence by pairwise comparison (Table 1). Furthermore, compared to amino acid sequences of maize LTP, rice LTP, Taiwan lily LfLTP and 'Nellie white' SCA (Figure 3), the results of alignment and similarity analysis showed that the sequence similarity with maize LTP was in range from lowest 54.84% to highest 56.99% and for rice LTP was from 50.55% to 52.75%, while Lilium longiflorum 'Nellie white' SCA was similar with Taiwan lily LfLTP, similarity reached the highest 98.90% and the lowest 92.31%, indicating that the sequence was homologous.
Although SCA amino acid sequence had high homology between the species, there were some obvious differences in the 22nd site, in which Easter lily, Taiwan lily and Asian lily were the valine (V), but Oriental lily was the glutamate (E) or glutamine (Q). While Easter lily, Taiwan lily and Asian lily were the alanine (A) in the 64th site, but Oriental lily was the asparagine (N). Likewise, Taiwan lily and Asian lily were the alanine (A) in the 71st site, Oriental lily was the glycine (G), Whereas SCA from Lilium longiflorum 'Nellie white' and LlSCA1 and LlSCA2 from the 'White heaven' in the 71st site were the alanine (A), LlSCA3 from 'White heaven' was the alanine (A), which was in line with SCA3 of three subtype of SCA isolated from Lilium longiflorum 'Nellie white' by Chae et al (2007) also was the alanine (A) the site of 82nd amino acids was the serine (S) in lily except for 'Nellie white' was the arginine (R) identified in GenBank. It remains to be verified whether the differences in the amino acids among the species occurs in the functional regions of protein resulting in the differences in pollen adhesion and influences on the directional pollen tube growth, then leading to generate the phenomenon of incompatibility.
In the sequence structure of the proteins, the cloned fragments contains the common structural features of LTP, including eight conserved cysteine residues and two conserved pentapeptide motifs (Thr/Ser-X1-X2-Asp-Arg/Lys and Pro-Tyr-X-Ile-Ser) (Douliez et al., 2000). However, the first amino acid of LaSCA4 and LlSCA3 was asparagine in the first motif instead of threonine or serine, which was consistent with the site of rice LTP. The second motif, the first amino acid of LlSCA1 was leucine instead of proline, there was only one difference in the site of amino acid between LlSCA1 and LlSCA2. According to nucleotide sequence analysis, we found that the 325th base in LlSCA1 was T while in LlSCA2 and LlSCA3 were the C. Nucleotide sequence analysis of LaSCA2 found that the 112nd nucleotide mutated from C to T leading to the fifth amino acid coding sequence transformed from CAG (glutamine) to TAG (stop codon), resulting in early translation termination. In order to further analyze the subsequent sites and to judge whether the conserved structural features of LTP occurred, the whole sequence still used to be the sequence alignment in Figure 3, the results found that the sequences behind the mutating bases had no any changed in encoding amino acids, so we preliminary judged that the mutagenesis on the site might have occurred, resulting in translation termination, and further speculated that LaSCA2 might be a pseudo-gene occurred during the evolution process of SCA . For maize LTP, the 46th of arginine and the 81st tyrosine were considered as the key sites where LTP functions with lipid molecules in vitro conditions (Han et al., 2001), indicating that the two sites had highly conservative in the sequence.
|
|
1.4 Phylogenetic tree construction and secondary structure prediction of SCA
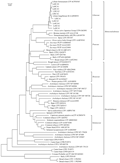
Ten amino acid sequence of Lily SCA except LaSCA2 and fifty seven known amino acid sequences of other plant LTP were employed to do alignment analysis to build the phylogenetic tree (Figure 4). It was found that the cloned ten sequences were clustered into a group with Lilium longiflorum 'Nellie white' SCA and the Taiwan lily LfLTP together, indicating that their evolutionary relationship should be the closest. The different SCA copies from the same variety can be well clustered together, Asian lily SCA and Taiwan lily LfLTP went into a group, while Easter lily 'White heaven' SCA and Easter lily 'Nellie white' SCA came to a group. The dendrogram also presented that the genetic distance between Asian Lily and Easter lily was closer than that between Asian lily and Oriental lilies, which might have certain correlations existing in the hybridizing between Easter lily and Asian lily easier than that between Oriental lily and Asia lily. Lily clustering into the group with the grass family belonging to monocotyledonous plant showed that this genetic relationship should be in line with classification in plants. Cluster analysis found that dicotyledonous plants of Rosaceae, Solanaceae, and cruciferous plants were clustered into a group to form branch, indicating that LTP genes during evolution should be relatively conservative. However, Arabidopsis LTPs including LTP6, LTP10, LTP8 and LTP11 were not clustered into the group with cruciferous plant in this dendrogram, while LTP9 had much closer genetic distance with the other LTPs, it may be due to Arabidopsis LTP encoding by a small multi-gene family (Arondel et al., 2000), resulting in differences in amino acid sequence of LTP proteins. Barley LTP2, wheat LTP2-1 and wheat LTP2-2 did not come together in the grass group, but went to form a single large branch with the cowpea nsLTP, which would be the reason that LTP can be divided into LTP â… and LTP â…¡ the size of the protein molecular weight. LTP â… located in the cell wall consisted by a 90-95 amino acid residues with the molecular weight of about 10 kDa, mainly existing in the aerial parts of plant tissues and organs, while LTP â…¡ consisted about 70 amino acid residues molecular weight of about 7kDa, mainly existing in the roots (Li et al, 2009). Cowpea nsLTP, barley LTP2, wheat LTP2-1 and wheat LTP2-2 belongs to type â…¡ LTP, other sequences belong to the type â… LTP.
Prediction of protein secondary structure was carried out (Figure 5) in this study, the results showed that there were also quite differences between the secondary structure of type â… and type â…¡ LTP. Type â… LTP has more α-helix than that of the type â…¡ LTP, while Type â… LTP has less β-turn than that of the type â…¡LTP. The overall difference of the secondary structure among the sequences of the type I LTP was not significant, α-helix and irregular coil are the main structural elements and four-stranded α-helix mainly distributed in the middle of the amino acid sequence, whereas irregular coiled form and extension mainly distributed in the end. In monocots, there was no β-turn appearing in the first α-helix region of SCA, and LTP protein, while in dicots, some β-turn structure existed in the region of LTP proteins. Based on the phylogenetic tree and the secondary structure prediction we might predict that the differentiation of plant LTP genes should occur before differentiation of monocots and dicots.
|
|
|
|
2 Discussions
SCA protein was first isolated from the stigma and style of Lilium longiflorum 'Nellie White' in Lord’s laboratory of the University of California in vivo observations by immunogold electron microscopy as well as in vitro adhesion assay have proved that SCA protein has adhesion functions to pollen tube (Park et al., 2000). We found that lily SCA gene had an open reading frame containing two exons and one intron. Regarding exons, the amino acid sequences deduced by the exons of SCAs of three lily species had high similarity, more than 80%, of which contained the conserved structural features of the LTP genes. However, there were significant amino acid differences occurring in the sites of the 22nd, 64th and 71st among species (Figure 3). Do the amino acid differences of these sites lead to change in the functions of SCA protein? Or do changes of these sites affect the specific recognition between the SCA protein and SCA receptors on the top plasma membrane pollen tube, and then affecting pollen tube adhesion and directional growth between different species, thus influence on the affinity of hybridization among the species? At present we are carrying out in vitro protein expression and purification of lily SCA genes of the different species as well as in vitro adhesion assay to determine the induction ability of the pollen tube adhesion and directional growth of the protein between interspecies and intra-species, accordingly, to determine the changes of these amino acid sites whether the SCA proteins are subject to cause the influences on pollen tube adhesion among lily species.
In introns, the locations of the intron of three species were quite conservative, but the lengths of the introns were differences. The homology of the introns was lower than that of the exons, there was little or a little difference existing in different copies of the introns of same species. Although intron does not encode protein, it plays an important regulatory role in gene expression. In most of cases, intron will enhance gene expression (Callis et al., 1987), of which the enhanced degree of gene expression would be related with many factors, such as characteristics of intron (Clancy and Hannah, 2002), features of promoter and exon sequence, intron positions on the vector (Wang, 2008), the sequence structure of the target gene, cell type and cell physiological status. Some introns have tissue-specific regulation to gene expression (Stemmler et al., 2005), while some introns have a disincentive function on gene expression (Hormuzdi et al., 1998). In this study, the intron sequences are quite differences existing in three lily species that might affect the expression of SCA, thereby leading to affect the recognizing capabilities of pollen tube to SCA in different species. Different copies of the SCA from the same species have differences in the introns, which might lead to be differential expressions of different copies of SCA in different tissues, accordingly resulting in different functions of different SCA copies of the same species, of which needs to be considered in the follow-up studies.
Our study also found that SCA has diversity existing in lily genome, which is consistent with the previous genetic findings of LTP gene in other species, such as Arabidopsis (Arondel et al., 2000; Soufleri et al., 1996; Chae et al., 2010). LTPs are considered as a kind of defense proteins as well as a class of sulfur protein-like stress-resistant peptides (Broekaert et al., 1997). Although stress resistance might be the dominant functions, these proteins might be recently considered to have additional function, that is, as the factor of chemotropism in signal transduction (Yang et al., 1999). Lily SCA has genetic diversity, it might belong to a multi-functional gene, nevertheless, this requires further biological validation.
3 Materials and Methods
3.1 for the test material
The bulbs of Asian lily 'Tresor' (Lilium Asiatic Hybrids), Easter lily 'White heaven' (Lilium Longiflorum Hybrids) and Oriental lily 'Caruso' (Lilium Oriental Hybrids) were purchased at the Beijing Shenzhou Kelaowo Horticultural Technology Co., Ltd., planted in the flower room at the tissue culture center of Beijing Agricultural College. Fresh young leaves without any harm of pests and diseases were collected into sealed plastic bag and stored in ultra-low temperature refrigerator at -80 ℃ ready for use.
2×Taq PCR Green Mix were purchased from Beijing Dingguo Changshun Biotechnology Co., Ltd. DNA markers, gel extraction kit and E. coli competent cells Top 10 were purchased from the Tiangen Company; T vector is the product of Takabo Bio-Engineering Co., Ltd.
3.2 Genomic DNA extraction
Genomic DNA was extracted following with the CTAB method (Lin, 2004).
3.3 Primer design and PCR amplification
The primers were designed according to non-coding region of the SCA cDNA of Lilium longiflorum 'Nellie white' from GenBank in NCBI, one of primer was LSCAF: 5'-ACTCCCATTCTTACCAGCTCTCCTT-3 'and another was LSCAR: 5'-CTGAAACAGAAGACTACAACACCGC-3' both synthesized by the Shanghai Yingjun Company.
In total of 25 μL volume of PCR reaction containing 2×Taq PCR Green Mix 12.5 μL, primers (10 μM) of 0.5 μL each, DNA template (35 ng/μL) 5 μL, adding ddH2O 6 μL up to 25 μL. PCR procedures were as following: 94℃ pre-denaturation for 5min and then 38 cycles of 94℃ denaturing for 40 s, 55℃ annealing for 30 s, 72℃ extension for 40 s, finally 72℃ extension for 10 min and placed at 4℃ for store. PCR products were scored by 2.0% agarose gel electrophoresis and photographed by gel imaging system camera.
3.4 Amplified fragment of SCA cloning, sequencing and aligning
PCR products were recovered from agarose gel and ligated to the pMD19-T vector and then transferred into competent Escherichia coli TOP 10 for cloning. Positive clones were picked by Blue-white screening method to be identified by inoculated medium PCR. The insert fragments of amplified products were determined by gel electrophoresis to be sequenced by Shanghai Yingjun Biotechnology Co., Ltd. for sequencing.
3.5 Sequence analysis
Similarity comparison of the cloned sequences was carried out by using DNAMAN software. The largest open reading frame was determined and the amino acid sequence was deduced by using DNAStar software. The possible existing signal peptide was predicted based on SignalP (http://www.cbs.dtu.dk/services/SignalP/) and the homologous sequences were searched online by using the NCBI BLAST program. The multiple comparisons of the sequences were conducted by using Clustal X and Mega4 as well as phylogenetic tree, was built by using neighbor-joining method. The secondary structures of proteins deduced from each DNA sequence were also predicted by SPOMA online analysis software.
Author's contributions
YJW is the executor who performed experimental design and experimental study as well as the analysis of data and results, wrote the manuscript. JTC and YHJ involved in experimental design and result analysis. KZZ is the principal investigator who conceived the project and took the responsible to supervise the experimental design, data analysis, paper preparation and revision. All authors have read and agreed the final context.
Acknowledgements
This project was jointly funded by the Natural Science Foundation of Beijing (6122004), the project of Science and Technology Innovation Platform of Beijing Municipal Educational Commission (PXM2009-014207-078529), the Beijing Nova Program (2003B013) and the project of the fund for enhancement of the level of scientific research of Beijing Municipal Education Commission. Authors appreciated the staffs in our laboratory who gave helpful guidance and comments for this research.
References
Arondel V., Vergnolle C., Cantrel C., and Kader J.C., 2000, Lipid transfer proteins are encoded by a small multigene family in Arabidopsis thaliana, Plant science, 157(1): 1-12
http://dx.doi.org/10.1016/S0168-9452(00)00232-6
Broekaert W.F., Cammue B.P.A., De Bolle M.F.C., Thevissen K., De Samblanx G.W., Osborn R., and Nielson D.K., 1997, Antimicrobial peptides from plants, Critical Reviews in Plant Science, 16(3): 297-323
http://dx.doi.org/10.1080/07352689709701952 http://dx.doi.org/10.1080/713608148
Chae K., Zhang K., Zhang L., Morikis D., Kim S.T., Mollet J., Rosa N., Tan K., and Lord E.M., 2007, Two SCA (Stima/style Cysteine-rich Adhesin) isoforms show structural differences that correlate with their levels of in vitro pollen tube adhesion activity, Journal of biological chemistry, 282(46): 33845-33858
http://dx.doi.org/10.1074/jbc.M703997200 PMid:17878166
Chae K., Gonong B.J., Kim S.C., Kieslich C.A., Morikis D., Balasubramanian S., and Lord E.M., 2010, A multifaceted study of stigma/style cysteine-rich adhesin (SCA)-like Arabidopsis lipid transfer proteins (LTPs) suggests diversified roles for these LTPs in plant growth and reproduction, Journal of Experimental Botany, 61(15): 4277-4290
http://dx.doi.org/10.1093/jxb/erq228 PMid:20667964 PMCid:2955742
Callis J., Fromm M., and Walbot V., 1987, Introns increase gene expression in cultured maize cells, Genes and Development, l(10): 1183-1200
http://dx.doi.org/10.1101/gad.1.10.1183
Clancy M., and Hannah L.C., 2002, Splicing of the maize Sh1 first intron is essential for enhancement of gene expression, and a T-rich motif increases expression without affecting splicing, Plant Physiol, 130(2): 918-929
http://dx.doi.org/10.1104/pp.008235 PMid:12376656 PMCid:166618
Douliez J.P., Michon T., Elmorjani K., and Marion D., 2000, Structure, biological and technological functions of lipid transfer proteins and indolines, the major lipid binding proteins from cereal kernels, Journal of cereal science, 32(1): 1-20
http://dx.doi.org/10.1006/jcrs.2000.0315
Han G.W., Lee J.Y., Song H.K., Chang C., Min K., Moon J., Shin D.H., Kopka M.L., Sawaya M.R., Yuan H.S., Kim T.D., Choe J., Lim D., Moon H.J., and Suh S.W., 2001, Structural basis of non-specific lipid binding in maize lipid-transfer protein complexes revealed by high-resolution X-ray crystallography, Journal of Molecular Biology, 308(2): 263-278
http://dx.doi.org/10.1006/jmbi.2001.4559 PMid:11327766
Higashiyama T., 2010, Peptide signaling in pollen–pistil interactions, Plant Cell Physiol., 51(2): 177-189
http://dx.doi.org/10.1093/pcp/pcq008 PMid:20081210
Hormuzdi S.G., Penttinen R., Jaenisch R. and Bornstein P., 1998, A gene targeting approach identifies a function for the first intron in expression of the α1 (I) collagen gene, Journal of Molecular Cell Biology, 18(6): 3368-3375
Kim S., Mollet J.C., Dong J., Zhang K., Park S.Y., and Lord E.M., 2003, Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism, Proc. Natl. Acad. Sci., USA, 100(26): 16125-16130
http://dx.doi.org/10.1073/pnas.2533800100 PMid:14671326 PMCid:307703
Li C.S., Zhao C.Z., and Li A.Q., Li X.F., Zhao G.J., and Wang X., 2009, Study of plant lipid transfer proteins, Shandong Agricultural Sciences, 8: 13-17
Lin J.S., eds., 2004, Modern cell and molecular biology techniques, Science Press, Beijing, China, pp.138-141
Mollet J.C., Park S.Y., Nothnagel E.A., and Lord E.M., 2000, A lily stylar pectin is necessary for pollen tube adhesion to an in vitro stylar matrix, Plant Cell, 12(9): 1737-1750
http://dx.doi.org/10.2307/3871186 PMid:11006344 PMCid:149082 http://dx.doi.org/10.1105/tpc.12.9.1737
Palanivelu R., and Preuss D., 2006, Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro, BMC Plant Bioogyl, 6: 7
http://dx.doi.org/10.1186/1471-2229-6-7 PMid:16595022 PMCid:1489931
Park S.Y., Jauh G.Y., Mollet J.C., Eckard K.J., Nothnagel E.A., Walling L.L., and Lord E.M., 2000, A lipid transfer-like protein is necessary for lily pollen tube adhesion to an in vitro stylar matrix, Plant Cell, 12(1): 151-164
http://dx.doi.org/10.2307/3871036 PMid:10634914 PMCid:140221 http://dx.doi.org/10.1105/tpc.12.1.151
Stemmler M.P., Hecht A., and Kemler R., 2005, E-cadherin intron-contains cis-regulatory elements essential for gene expression, Development, 132(5): 965-976
http://dx.doi.org/10.1242/dev.01662 PMid:15673570
Soufleri I.A., Vergnolle C., Miginiac E., and Kader J.C., 1996, Germination-specific lipid transfer protein cDNAs in Brassica napus L., Planta, 199(2): 229-237
http://dx.doi.org/10.1007/BF00196563 PMid:8680310
Van Tuyl J.M., Van Diën M.P., Van Creij M.G.M., Van Kleinwee T.C.M., Franken J., and Bino R.J., 1991, Application of in vitro pollination, ovary culture, ovule culture and embryo rescue for overcoming incongruity barriers in interspecific Lilium crosses, Plant Science, 74(1): 115-126
http://dx.doi.org/10.1016/0168-9452(91)90262-7
Van Tuyl J.M., Van Dijken A., Chi H.S., Lim K.B., Villemoes S., and Van Kronenburg B.C.E., 2000, Breakthroughs in interspecific hybridization of lily, Acta Horticulturae, 508: 83-88
Wang Y.B., 2008, Intron-mediated enhancement of the expression of bt cry1Ah gene in transgenic maize,Dissertation for Ph.D., Biotechnology Research Institute CAAS, Supervisor:Huang D.F., pp.15-20
Yang D., Chertov O., Bykovskaia S.N., Chen Q., Buffo M.J., Shogan J., Anderson M., Schröder J.M., Wang J.M., Howard O.M.Z., and Oppenheim J.J., 1999, β-Defensins: linking innate and adaptive immunity through dendritic and T cell CCR6, Science, 286(5439): 525-528
http://dx.doi.org/10.1126/science.286.5439.525 PMid:10521347
Yubero-Serrano E.M., Enriqueta M., Nieves M.E., Blanco J.M., and Caballero J.L., 2003, Identification of a strawberry gene encoding a non-specific lipid transfer protein that responds to ABA, wounding and cold stress, Journal of Experimental Botany, 54(389): 1865-1877
http://dx.doi.org/10.1093/jxb/erg211 PMid:12869521
. PDF(1390KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Yuejuan Wu
. Jinteng Cui
. Kezhong Zhang
. Yuehui Jia
Related articles
. Lily ( Lilium spp. )
. Pollen tube adhesion
. Directional growth
. SCA
. Gene diversity
Tools
. Email to a friend
. Post a comment