Research Article
Study of Dichlorophenoxyacetic Acid and 6-Benzylaminopurine Effects on Callus Development in Cucurbita moschata 
2 Kunming Institute of Botany, University of Chinese Academy of Sciences, China
3 Centre of Excellence in Molecular Biology, University of the Punjab Lahore, Pakistan
 Author
Author  Correspondence author
Correspondence author
Molecular Plant Breeding, 2016, Vol. 7, No. 3 doi: 10.5376/mpb.2016.07.0003
Received: 07 Nov., 2015 Accepted: 22 Dec., 2015 Published: 02 Jan., 2016
Saba Riaz, Athar Hussian Shah, Saif-ul-Malook, and Qurban Ali, 2016, Study of Dichlorophenoxyacetic acid and 6-Benzylaminopurine effects on callus development in Cucurbita moschata, Molecular Plant Breeding, 7(03): 1-12 (doi: 10.5376/mpb.2016.07.0003)
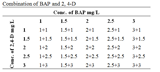
Cucurbita moschata is an important and popular vegetable, used throughout the world due to its taste, nutritional and medicinal values. Plant growth hormones play an important role in the growth and development of crop plants. Callus development of Cucurbita moschata was accessed produced from cotyledon, leaf, hypocotyls and root under the application of auxins dichlorophenoxyacetic acid (2,4-D) and cytokinins 6-Benzylaminopurine (BAP) with different concentrations. It was found that 1.5mg/L BAP+1.5mg/L 2,4-D and 0.5mg/L BAP+1.5mg/L 2,4-D combination was best for callus development from cotyledon, as both gave a callus index of 300 but the time for callusing was 9 and 10 days respectively vs. 2,4-D and BAP (3 mg/L) for callus induction by leaves, 2mg/L BAP+1.5mg/L 2,4-D for callus induction from hypocotyl and from root the combination of 1.5mg/L BAP +3mg/L 2,4-D produced the best results. It was suggested form the results that the combination of optimum concentrations (1.5-3mg/L) of BAP and 2, 4-D may be useful for developing higher callus mass in short time.
 |
.png) Table 1 Effect of different concentrations of BAP and 2,4-D for callus developed from cotyledon of C. moschata Note: All these values are sum means of three parallel replicates in which ± indicates standard error among the values, which differ significantly at p ≤ 0.05. The optimum value of Duncan for days to callusing is significant of these results in terms of statistical analysis. |
 Figure 1 Brown and compact callus developed from cotyledon of C. moschata in medium MS + 0.5 mg/L BAP and 1.5 mg/L 2,4-D (22 days old) (1x) |
 Figure 2 Whitish Yellow and granular callus developed from cotyledon of C. moschata in medium MS + 1.5 mg/L BAP and 1.5 mg/L 2,4-D (20 days old) (1x) |
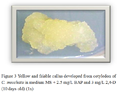 Figure 3 Yellow and friable callus developed from cotyledon of C. moschata in medium MS + 2.5 mg/L BAP and 3 mg/L 2,4-D (10 days old) (1x) |
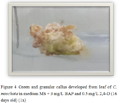 Figure 4 Green and granular callus developed from leaf of C. moschata in medium MS + 3 mg/L BAP and 0.5 mg/L 2,4-D (16 days old) (1x) |
 Figure 5 Light brown and compact callus developed from leaf of C. moschata in medium MS + 2.5 mg/L BAP and 3 mg/L 2,4-D (12 days old) (1x) |
.png) Table 2 Effect of different concentrations of BAP and 2,4-D for callus developed from leaf of C. moschata Note: All these values are sum means of three parallel replicates in which ± indicates standard error among the values, which differ significantly at p ≤ 0.05. The optimum value of Duncan for days to callusing is significant of these results in terms of statistical analysis. |
.png) Table 3 Effect of different concentrations of BAP and 2, 4-D for callus developed from hypocotyl of C. Note: All these values are sum means of three parallel replicates in which ± indicates standard error among the values, which differ significantly at p ≤ 0.05. The optimum value of Duncan for days to callusing is insignificant of these results in terms of statistical analysis |
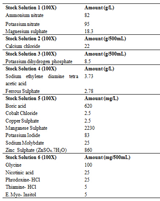
Yellow callus turned to dark brown after sub-culturing. 3mg/L BAP+0.5mg/L 2,4-D induced the amount of copious yellow green callus. Copious yellow brown compact callus was produced in Conc. of 1mg/L BAP +1mg/L 2,4-D which was having 17 days. 1.5mg/L BAP +1mg/L 2,4-D formed yellow copious callus in 11 days. Copious amount of yellow green callus was observed in 2.5mg/L BAP and 1mg/L 2,4-D. Granular light brown callus was produced by 3mg/L BAP +1mg/L 2,4-D in 13 days. The concentration of 0.5mg/L BAP +1.5mg/L 2,4-D gave light yellow colour callus that was granular in texture, callus induction percent was 100%. Copious amount callus has been produced by 1mg/L BAP +1.5mg/L 2,4-D with yellow colour callus. The callus texture was granular. Duration for callus initiation was 12 days. 1.5mg/L BAP +1.5mg/L 2,4-D formed green callus in 13 days. 2mg/L BAP +1.5mg/L 2,4-D combination produced copious mass of callus of green brown colour (Figure 6). Conc. of 0.5mg/L BAP +2mg/L 2,4-D formed copious amount of callus and colour was whitish green. 1mg/L BAP +2mg/L 2,4-D formed brown colour of callus with 100% callus induction. Callus development occurred in 11 days (Figure 7). Moderate amount of callus was produced in the Conc. of 1.5mg/L BAP +2mg/L 2,4-D. callusing occurred in 13 days and yellow brown granular callus formed. Induction of callus was 67% and callus index found was 133. Callus induction with combination 2mg/L BAP +2mg/L 2,4-D was in copious amount. The callus was yellow and compact in 12 days. Light yellow callus were produced with 2.5 mg/L BAP +2mg/L 2,4-D. The callus response percentage was 100%, produced in 14 days. Copious amount of callus growth was found with Conc. of 1mg/L BAP +2.5mg/L 2,4-D that has callus yellow in colour. Yellow callus formed under effect of 0.5mg/L BAP +3mg/L 2,4-D and 100% explants produced callus. 1.5mg/L BAP+3mg/L 2,4-D formed less amount callus of yellow green colour. 2.5mg/L BAP +3mg/L 2,4-D found copious amount of callus in 11 days. Ashutosh et al., (2012) examined highest development of total alkaloid contented was found in and 1mg/L BAP +0.5mg/L 2, 4-D associated with other amalgamations. Everaldo and Anthony, (2001) reported that the use of BAP and 2, 4-D caused the improvement in the plant body weight and growth of the plants. Oswell et al., (2007); Ali et al., (2014); Qamar et al., (2014); Butt et al., (2015) reported similar results. Present results were in conformity with the results of Alsop et al., (1978) and Punja et al., (1990) in which callus induction from hypocotyl explants was induced.
 Figure 6 Greenish brown and granular callus developed from hypoctyl of C. moschata in medium MS + 2 mg/L BAP and 1.5 mg/L 2,4-D (11 days old) (1x) |
 Figure 7 Brown and friable callus developed from hypocotyl of C. moschata in medium MS + 1 mg/L BAP and 2 mg/L 2,4-D (22 days old) (1x) |
.png) Table 4 Effect of different concentrations of BAP and 2,4-D for callus developed from root of C. moschata Note: All these values are sum means of three parallel replicates in which ± indicates standard error among the values, which differ significantly at p ≤ 0.05. The optimum value of Duncan for days to callusing is significant of these results in terms of statistical analysis. |
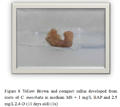 Figure 8 Yellow Brown and compact callus developed from roots of C. moschata in medium MS + 1 mg/L BAP and 2.5 mg/L 2,4-D (10 days old) (1x) |
 Figure 9 Light brown and compact callus developed from root of C. moschata in medium MS + 1.5 mg/L BAP and 3 mg/L 2,4-D (12 days old) (1x) |
 Figure 10 Brown and compact callus developed from root of C. moschata in medium MS + 2 mg/L BAP and 3 mg/L 2,4-D (13 days old) (1x) |
. PDF(535KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Saba Riaz
. Athar Hussian Shah
. Saif-ul- Malook
. Qurban Ali
Related articles
. Cucurbita moschata
. Callus
. Dichlorophenoxyacetic acid
. 6-Benzylaminopurine
. Plant growth regulators
Tools
. Email to a friend
. Post a comment