2 Department of plant sciences, University of California Davis, CA, USA
 Author
Author  Correspondence author
Correspondence author
Molecular Plant Breeding, 2016, Vol. 7, No. 16 doi: 10.5376/mpb.2016.07.0016
Received: 20 Dec., 2015 Accepted: 19 Feb., 2016 Published: 19 Feb., 2016
Siddra Ijaz, 2016, Molecular Biology of Ethylene, a Review, Molecular Plant Breeding, 7(11): 1-7 (doi: 10.5376/mpb.2016.07.0011)
Ethylene is a plant hormone of wide-ranging diverse effects on plants physiology and development. This simple molecule interacts with other hormones and factors with very versatile and fantastic manner for executing growth as well as developmental events in plants. Ethylene has strong impact on myriad developmental events of plants from germination to senescence. Here intent is to focus and highlight the importance, synthesis, regulatory genetic network and signaling integration of this versatile molecule that affects plant growth and development in a myriad ways.
Introduction
Ethylene is a plant hormone and simplest unsaturated hydrocarbon. It is a gaseous signal molecule and a wide range of metabolic, physiological as well as developmental events in plants is regulated by this biologically active gas (Schaller, 2012). Ethylene has strong impact and effect on climacteric fruits ripening and also on triple responses in etiolated seedling of dicotyledonous plants. Fruit ripening mechanism is a complex and genetically programmed developmental event. Ripening process in fruits is started from its one sector and spreads to adjoining sector. In this process ethylene is diffused cell to cell and thereby is assimilated in whole fruit. This physiological event is culminated in remarkable variations and modification in texture, color, aroma and flavor of fruit flesh (Klee and Giovannoni, 2011).
Ripening mechanism is different in different fruits; therefore on the basis respiration rate and ethylene production, these are grouped into two categories; climacteric and non-climacteric fruits. The former ones show rise in respiratory rate and concomitant ethylene production at high level when they close to maturity and edibility. At this time ethylene burst is achieved. Thus for ripening climacteric fruits have no need to remain attached to tree and they ripen after harvesting such as banana, mango, apple, tomato, papaya etc. Whereas in non-climacteric fruits such as citrus, grapes, strawberries, and pineapple etc, no ethylene burst is achieved at this stage and respiration rate is at its lowest level and is continuously declined until senescence occurs. Hence these fruits only ripen when they are on trees or vines. In climacteric fruits, for operating ethylene regulation, two systems have been proposed i-e., ethylene autoinhibitory and ethylene autocatalytic. During normal vegetative growth, former one is functional and is also involved in the ethylene production at basal level that are noticed in all tissues of climacteric fruits and even in non-climacteric fruits. While later one is operated during climacteric fruit ripening and is also responsible in the senescence of some petals (Alexander and Grierson, 2002).
Pollination stimulates ethylene induction and production in floral organ that leads to growth of ovaries and floral senescence and abscission (Balbi and Lomax, 2003; Wang et al., 2005). Genes involved in ethylene biosynthetic pathway as well as responsible for its signaling are down regulated after fruit setting (Pandolfini et al., 2007; Stepanova et al., 2008) when plants are pollinated and even treated with gibberellin. The effect of ethylene in fruit setting and development was not under keen consideration of scientists and that’s why had not been studied eagerly until last 1-2 decades. Now research on ethylene biochemistry, molecular biology and physiology has been flourishing. The extracted knowledge of studies on ethylene synthesis in plants and its role and impact in plant physiology and development has advantageous and beneficial to various agricultural systems and the trend is advancing and expediting.
1 Biosynthetic Pathway of Ethylene
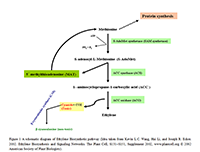
Ethylene biosynthetic pathway is entrenched in higher plants (Bleecker and Kende, 2000). Biosynthetic and signaling pathways of ethylene have been studying extensively for last few decades. These pathways have gained much attention in the area of plant hormone physiology (Kende, 1993). Major breakthrough in this subject came through the identification of S-adenosyl-L-methionine (S-AdoMet) and 1-aminocyclopropane-1-carboxylic acid (ACC), as ethylene precursors (Yang and Hoffman, 1984). ACC, a non-protein cyclic compound is produced from S-AdoMet in a reaction carried out by a specialized enzyme ACC synthase (ACS) (Kende, 1993). However, S-adenosyl-L-methionine is formed from methionine via S-AdoMet synthetase (SAM synthetase) enzyme at the cost of ATP (Ravanel et al., 1998).
Methionine is not only the essential brick of protein building while about 80% of cellular methionine is also involved in the formation of S-AdoMet (prime methyl donor in plants) (Kevin et al., 2002). In the first committed step of ethylene biosynthetic pathway, besides ACC, another compound 5´-methyl- thioadenosine (MAT) is also formed that is subsequently converted into methionine via modified methionine cycle (Bleecker and Kende, 2000). As intermediate compound of ethylene synthesis pathway, ACC is then being used as substrate of another enzyme of this route, ACC oxidase (ACO). This enzyme mediates the reaction of ethylene formation from ACC that is the immediate precursor of ethylene (Yang and Hoffman, 1984).
Modified methionine cycle is a type of salvage pathway that keeps and save methylthio group from every round and for another cycle of ethylene production at the expense of one ATP molecule. Thereby ethylene can be synthesized even at high rate, when there is scarcity of methionine in a cell (Bleecker, 2000 and Kevin et al., 2002). In addition to ethylene, cyanide and CO2, are also the end products of this biological route. As cyanide is toxic, thus cell has to flush out this compound, as sake of its survival. Therefore by the activity of β-cyanoalanine synthase (CAS) that detoxifies cyanide and converts it into β-cyanoalanine, a non-toxic compound, cell is prevented to toxicity (Kevin et al., 2002) (Figure 1).
|
Figure 1 A schematic diagram of Ethylene Biosynthetic pathway (Idea taken from Kevin L.C. Wang, Hai Li, and Joseph R. Ecker. 2002. Ethylene Biosynthesis and Signaling Networks. The Plant Cell, S131–S151, Supplement 2002, www.plantcell.org © 2002 American Society of Plant Biologists). |
2 Regulation of Ethylene Biosynthesis Related Genes
In plants, progress in ripening stage had proved to be linked with the induction and upregulation of various genes. Hence involvement of ethylene dependent and ethylene independent gene regulations have been observed using molecular and expression analyses of ripening related genes by developing mutants and transgenic plants (Picton et al., 1993 and Theologis et al., 1993). Impact of positive as well as negative feedback regulation on ethylene biosyntheses is also well established (Barry et al., 2000). Genes encoding ACC synthase and ACC oxidase belong to small multigene families. The differential expression of these enzymes is regulated through, hormonal, environmental d developmental stimuli or signals (Zarembinski and Theologis, 1994 and Barry et al., 2000). A complex regulatory network comprising environmental and developmental signals is existed for regulating the expression of genes involved in biosynthetic pathway of ethylene (Johnson and Ecker, 1998). Regulation of ACC synthase gene is based on the expression of this gene in response to internal and external cues. Various isoforms of ACS is present such as in tomato, Le-ACS2, Le-ACS4 and Le-ACS6. However their feedback regulation is different when ethylene is synthesized during fruit ripening thereby former two are positively regulated whereas last one is negatively regulated by ethylene synthesis at this stage (Nakatsuka et al., 1998). The only common feature of these isoforms of ACS gene is their spatial and temporal regulation by various endogenous as well as exogenous stimuli or signals.
3 Ethylene Regulated Genes
The mechanism of ethylene-regulated genes has unraveled by molecular characterization of the promoter regions of ripening-related genes and thereby an ethylene-responsive element containing 8 bp motifs (A (A/T) TTCAAA) has been identified (Montgomery et al., 1993 and Itzhaki et al., 1994).
Similarly, two ethylene regulated genes, E4 and ER69 have been identified (Zegzouti et al., 1999) and these both genes are involved also in methionine cycle, encoding methionine sulphoxide reductase protein and cobalamine-independent methionine synthase respectively (Montgomery et al., 1993 and Zegzouti et al., 1999). In addition to E4, another gene E8 is also ethylene dependent for its induction (Lincoln et al., 1987). Although expression of E4 gene is induced in leaves in response to ethylene while it is not in case of E8 gene, predicting that their ethylene regulation is tissue specific and even developmentally regulated (Lincoln and Fischer, 1988).
4 Effect of Ethylene on Plant Physiology
Ethylene has crucial impact on the growth and development of plant. This simple, gaseous plant hormone affects and regulates the cell division, cell size and cell differentiation; thereby it has major effect on plant physiology. Plant growth and developmental changes are synchronized and under hormonal control. Therefore any mutation in ethylene synthesis related genes as well as ethylene signaling related genes would affect and alter the timing of plant developmental processes, thus this type of mutation is heterchronic mutation (Tsuchisaka and Theologis, 2004).
Ethylene modulates and alters developmental processes and growth dynamics such as seed germination, growth and development of plants organs, ripening and maturing as well as organs senescence and abscission (Schaller and Kieber, 2002). Ethylene induces and control root induction and formation, root nodules formation in leguminous crops, restricts the development of bulbs and tubers, stimulates the formation of female part instead of male part in family cucurbitaceae, similarly in some plants species ethylene promote flower induction while in others, it’s otherwise.
In plants, all biotic and abiotic factors are elicited ethylene synthesis. Regulation of ethylene synthesis is highly influenced by various developmental signals and hormones including auxin, cytokinins, and gibberellin etc (Figure 2). This is also intensively enhanced by environmental and biological factors vsuch as drought, salinity, cold, pathogens as well as insect pests etc (Bleecker and Kende, 2000). This shows the key role of ethylene in internal growth coordination and even in defense mechanism for survival against environmental confronts. Up-and- coming evidences have unraveled that ethylene sensitivity varies in diverse tissues and divergent developmental stages in consequence of signaling interactions with other plant hormones, metabolites and environmental cues (Klee, 2004; Alonso and Stepanova, 2004). Current studies in Arabidopsis thaliana by developing its ethylene mutants have also revealed an interconnection between early ovule abortion and silique size (Guo and Ecker, 2004).
|
Figure 2 Ethylene Signaling pathway: a schematic diagram of ethylene signaling transduction pathway depicting the signal cascade controlling infantile to mature phase transition (Idea taken from Schaller, E. G. 2012. Ethylene and the regulation of plant development. BMC Biology, 10:9). |
5 Role of Ethylene in Biotic Stresses in Plants
Ethylene significant role in plant defense depicts its association in multiple physiological processes by stimulating necrosis and establishing hypersensitive response in plants (Lund et al., 1998 and Ciardi et al., 2001). Plant defense related dynamics including phytoalexins induction, production pathogenesis related proteins as well as cell wall modulations are activated by ethylene (Tornero et al., 1997 and Fan et al., 2000). Hence ethylene has been an object of intensive study regarding resistance pathways and mechanisms from last few decades. The role of ethylene in resistance against pathogens had been studied by Hoffman et al., (1999) by developing soybean ethylene insensitive mutants. They observed that ethylene helps in restricting the pathogen invasion by leaf abscission when disease is aggravated. Similar studies had been done on Arabidopsis thaliana and Nicotiana tabacum (Knoester et al., 1998 and Thomma et al., 1999) by developing their mutants to explore the versatile role of ethylene in plant defense against pathogens.
Immature phase to Mature phase transition
Figure 3 Ethylene Signaling pathway: a schematic diagram of ethylene signaling transduction pathway depicting the signal cascade controlling infantile to mature phase transition (Idea taken from Schaller, E. G. 2012. Ethylene and the regulation of plant development. BMC Biology, 10:9).
6 Ethylene Perception and Signaling Pathway
Plants have diverse mechanisms for modulating themselves and showing elasticity against altering internal and external environmental conditions. Among these, ethylene signaling pathway is at imperative status that plants have espoused for regulating stress responses. The foremost elements in ethylene signaling pathway comprise the ethylene receptors, the Raf-like serine/threonine kinase, CTR1, EIN2 (transmembrane protein), and the EIN3-like (EIL) family of transcription factors (Alonso et al., 1999).
A linear ethylene signal pathway has been recognized based on genetic and molecular analyses of ethylene response mutants starting from hormone perception at the membrane to transcriptional regulation in the nucleus (Bleecker and Kende, 2000 ; Chang and Stadler, 2001). Ethylene is sensed by transmembrane receptors. CTR1 as a negative regulator of this pathway is present downstream to receptors. Downstream of CRT1, ethylene receptors EIN2 and EIN3, EIN5, and EIN6 are present as positive regulators while EIN3 intercede transcriptional cascade of ethylene-regulated genes (Chen et al., 2005; Guo and Ecker, 2004). There are various families of ethylene induced genes that are present downstream of EIN3 like transcription factors (EIL) or proteins including ethylene response factor (ERF) and ethylene response DNA-binding factor (EDF) that signifying and demonstrating the transcription cascade that works downstream of EIL proteins (Figure 3). On the basis sequence and structural similarities, ethylene receptors present in different plant species are categorized into two groups. Group I receptors consists of ETR1 and ERSI whereas ETR2, EIN4, and ERS2 are the members of group II receptors in arabidopsis. Ethylene on binding to receptors leads to the inactivation of these receptors (Hua and Meyerowitz, 1998). Thus absence of ethylene supposed to be the basis of the activation of CTRI that is the negative regulator of ethylene signaling pathway (Kieber et al., 1993). Binding of ethylene to its receptors is allied with its co-factor that is copper, which is carried by the copper transporter RAN (Tuteja, 2007).
|
Figure 3 Ethylene synthesis and signaling are influenced by multiple factors including Abiotic and biotic factors, developmental cues and plant hormones that alter plant processes from germination to senescence. (Idea taken from Yoo SD, Cho Y, Sheen J. 2009. Emerging connections in the ethylene signaling network. Trends Plant Sci. 14(5):270-9. doi: 10.1016/j.tplants.2009.02.007. Epub 2009 Apr 15.) |
Although clear functional attributes of EIN2, EIN5, and EIN6 at molecular level has not been cleared yet. However EIN2 is an integral membrane protein while EIN3 is a nuclear protein and as a transcription factor it regulates the expression of its immediate target genes like Ethylene Response Factor1 (ERF1) (Alonso et al., 1999; Chao et al., 1997 and Solano et al., 1998). This factor belongs to a large family of transcription factors containing APETALA2 domain. Transcription factors of this family GCC box, present in ethylene inducible gene’s promoters (Hao et al., 1998). Thus, a transcriptional cascade that is interceded by EIN3/EIN3-like (EIL) and Ethylene Response Factor1 escorts the ethylene controlled gene expression regulation.
By using molecular genetics and bioinformatics tools prime signaling components of ethylene pathway has been identified in Arabidopsis thaliana that are multiple membrane receptors, nuclear transcription factor families, an intracellular signaling protein kinase (PK), a membrane transporter-like regulator and F-box proteins (Johnson and Ecker, 1998; Chang et al., 1993; Solano et al., 1998). The existence and identifications of these key ethylene related regulators in about all plant species has allude to highly conserved nature of this ethylene signaling pathway that have developed for functioning in sundry lifestyles and developmental programs.
7 Outlook
Ethylene as a gaseous phytohormone has been attaining great interest of plant scientists for few decades. Ethylene signaling, perception and integration of identified genetic components and regulators into mechanistic proceedings and events at genetic as well as molecular level are the current challenges. For elucidating and expliciting the ethylene functional role in signaling pathway, the direct and specific regulatory components of ethylene linked networks should be distinguished from indirect, shared or general regulators as well as mediators. Understanding of molecular interaction of ethylene with other plant hormones is a novel aspect for plant physiologists that unravel unique underlying mechanisms. This would explicit ethylene related responses that drive coordination and interaction of endogenous developmental routes as well as internal programs with external environmental signals or stimuli for modulating and adapting plant’s physiology for its survival and growth.
Alonso J.M., Hirayama T., Roman G., Nourizadeh S., Ecker J.R., 1999, EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis, Science, 284: 2148-2152
http://dx.doi.org/10.1126/science.284.5423.2148
Alonso J.M., and Stepanova A.N., 2004, The ethylene signaling pathway, Science 306: 1513–1515
http://dx.doi.org/10.1126/science.1104812
Balbi V., and Lomax T.L., 2003, Regulation of early tomato fruit development by the Diageotropica gene, Plant Physiol, 131: 186–197
http://dx.doi.org/10.1104/pp.010132
Barry C.S., Llop-Tous M.I., and Grierson D., 2000, The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato, Plant Physiol. ,123: 979–986
http://dx.doi.org/10.1104/pp.123.3.979
Bleecker A.B., and Kende H., 2000, Ethylene: a gaseous signal molecule in plants, Annu Rev Cell Dev Biol, 16: 1-18
http://dx.doi.org/10.1146/annurev.cellbio.16.1.1
Bleecker A.B., 2000, ETHYLENE: A Gaseous Signal Molecule in Plants, Annu. Rev. Cell Dev. Biol., 16: 1–18
http://dx.doi.org/10.1146/annurev.cellbio.16.1.1
Bleecker A.B., and Kende H., 2000. Ethylene: A gaseous signal molecule in plants, Annu. Rev. Cell Dev. Biol., 16: 1–18
http://dx.doi.org/10.1146/annurev.cellbio.16.1.1
Chang C., Kwok S.F., Bleecker A.B., and Meyerowitz E.M., 1993, Arabidopsis ethylene-response gene ETR1: similarity of product to two component regulators, Science, 262: 539-544
http://dx.doi.org/10.1126/science.8211181
Chang C., and Stadler, 2001, Ethylene hormone receptor action in Arabidopsis, Bioessays, 23: 619-627
http://dx.doi.org/10.1002/bies.1087
Chao Q., Rothenberg M., Solano R., Roman G., Terzaghi W., and Ecker J.R., 1997, Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins, Cell, 89: 1133-1144
http://dx.doi.org/10.1016/S0092-8674(00)80300-1
Chen Y.F., Etheridge N., and Schaller G.E., 2005, Ethylene signaling transduction, Ann. Bot., 95: 901-15
http://dx.doi.org/10.1093/aob/mci100
Ciardi J.A., Tieman D.M., Jones J.B., and Klee H.J., 2001, Reduced expression of the tomato ethylene receptor gene LeETR4 enhances the hypersensitive response to Xanthomonas campestris pv. Vesicatoria, Mol Plant-Microbe Interact, 14: 487–495
http://dx.doi.org/10.1094/MPMI.2001.14.4.487
Fan X.T., Mattheis J.P., and Roberts R.G., 2000, Biosynthesis of phytoalexin in carrot root requires ethylene action, Physiol Plant, 110: 450–454
http://dx.doi.org/10.1111/j.1399-3054.2000.1100404.x
Guo H., and Ecker J.R., 2004, The ethylene signaling pathway: new insights, Curr Opin Plant Biol, 7: 40-9
http://dx.doi.org/10.1016/j.pbi.2003.11.011
Hao D., Ohme-Takagi M., and Sarai A., 1998, Unique mode of GCC box recognition by the DNA-binding domain of ethylene-responsive element-binding factor (ERF domain) in plants, J Biol Chem, 273: 26857-26861
http://dx.doi.org/10.1074/jbc.273.41.26857
Hoffman T., Schmidt J.C., Zheng X., and Bent A.F., 1999, Isolation of ethylene-insensitive soybean mutants that are altered in pathogen susceptibility and gene-for-gene disease resistance, Plant Physiol., 119: 935–49
http://dx.doi.org/10.1104/pp.119.3.935
Hua J., and Meyerowitz E.M., 1998, Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana, Cell, 94: 261-271
http://dx.doi.org/10.1016/S0092-8674(00)81425-7
Itzhaki H., Maxson J.M., Woodson W.R., 1994, An ethylene responsive enhancer element is involved in the senescence-related expression of the carnation glutathione-S-transferase (GST1) gene, Proceedings of the National Academy of Sciences, USA, 91: 8925-8929
http://dx.doi.org/10.1073/pnas.91.19.8925
Johnson P.R., and Ecker J.R., 1998, The ethylene gas signal transduction pathway: a molecular perspective, Annu Rev Genet, 32: 227-254
http://dx.doi.org/10.1146/annurev.genet.32.1.227
Johnson P.R., and Ecker, J.R. 1998, The ethylene gas signal transduction pathway: A molecular perspective, Annu. Rev.Genet., 32: 227–254
http://dx.doi.org/10.1146/annurev.genet.32.1.227
Kende H., 1993, Ethylene biosynthesis, Annual Review of Plant Physiology and Plant Molecular Biology, 44: 283-307
http://dx.doi.org/10.1146/annurev.pp.44.060193.001435
Kevin L.C., Wang H.Li., and Joseph R.E., 2002, Ethylene biosynthesis and signaling networks, The Plant Cell, S131–S151, Supplement 2002, www.plantcell.org © 2002 American Society of Plant Biologists
Kieber J.J., Rothenberg M., Roman G., Feldmann K.A., Ecker J.R., 1993, CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases, Cell 1993, 72: 427-441
http://dx.doi.org/10.1016/0092-8674(93)90119-B
Klee, H.J. and Giovannoni, J.J., 2011, Genetics and control of tomato fruit ripening and quality attributes, Annu Rev Genet, 45:41-59 doi: 10.1146/annurev-genet-110410-132507
http://dx.doi.org/10.1146/annurev-genet-110410-132507
Klee H.J., 2004, Ethylene signal transduction, Moving beyond Arabidopsis, Plant Physiol, 135: 660–667
http://dx.doi.org/10.1104/pp.104.040998
Knoester M., van Loon L.C., J. van den Heuvel J., Hennig J., Bol J.F., and Linthorst H.J., 1998, Ethylene-insensitive tobacco lacks non-host resistance against soil-borne fungi, Proc. Natl. Acad. Sci., 95: 1933-1937
http://dx.doi.org/10.1073/pnas.95.4.1933
Lincoln J.E., Cordes S., Read E., and Fischer R.L., 1987, Regulation of gene expression ethylene during Lycopersicon esculentum (tomato) fruit development, Proceedings of the National Academy of Sciences, USA, 84: 2793-2797
http://dx.doi.org/10.1073/pnas.84.9.2793
Lincoln J.E., and Fischer R.L., 1988. Regulation of gene expression by ethylene in wild-type and rin tomato fruit (Lycopersicon esculentum) fruit, Plant Physiology, 88: 370-374
http://dx.doi.org/10.1104/pp.88.2.370
Lucille A., and Don G., 2002, Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening, Journal of Experimental Botany, 53(377): 2039-2055
http://dx.doi.org/10.1093/jxb/erf072
Lund S.T., Stall R.E., and Klee H.J., 1998, Ethylene regulates the susceptible response to pathogen infection in tomato, Plant Cell, 10: 371–382
http://dx.doi.org/10.1105/tpc.10.3.371
Montgomery J., Goldman S., Deikman J., Margossian L., and Fischer R.L., 1993, Identification of an ethylene-responsive region in the promoter of a fruit ripening gene, Proceedings of the National Academy of Sciences, USA, 90: 5939-5943
http://dx.doi.org/10.1073/pnas.90.13.5939
Nakatsuka A., Murachi S., Okunishi H., Shiomi S., Nakano R., Kubo Y., and Inaba A., 1998, Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening, Plant Physiol, 118: 1295–1305
http://dx.doi.org/10.1104/pp.118.4.1295
Pandolfini T., Molesini B., and Spena A., 2007, Molecular dissection of the role of auxin in fruit initiation, Trends Plant Sci, 12: 327–329
http://dx.doi.org/10.1016/j.tplants.2007.06.011
Picton S., Barton S.L., Bouzayen M., Hamilton A.J., and Grierson D., 1993, Altered fruit ripening and leaf senescence in tomatoes expressing an antisense ethylene-forming enzyme transgene, The Plant Journal, 3: 469-481
http://dx.doi.org/10.1111/j.1365-313X.1993.tb00167.x
Ravanel S., Gakiere B., Job D., and Douce R., 1998, The specific features of methionine biosynthesis and metabolism in plants, Proc. Natl. Acad. Sci., USA, 95: 7805–7812
http://dx.doi.org/10.1073/pnas.95.13.7805
Schaller E.G., 2012, Ethylene and the regulation of plant development, BMC Biology, 10: 9.
http://dx.doi.org/10.1186/1741-7007-10-9
Schaller G.E., and Kieber J.J, 2002, Ethylene. In The Arabidopsis Book, American Society of Plant Biologists DOI: 10.1199/tab.0071
http://dx.doi.org/10.1199/tab.0071
Solano R., Stepanova A., Chao Q., and Ecker J.R., 1998, Nuclear events in ethylene signaling: a transcriptional cascade mediated by ethylene-insensitive3 and ethylene-responsefactor1, Genes Dev, 12: 3703-3714
http://dx.doi.org/10.1101/gad.12.23.3703
Stepanova A.N., Robertson-Hoyt J., Yun J., Benavente L.M., Xie D., Doležal K., chlereth A., Jürgens G., and Alonso J.M., 2008, TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development, Cell, 133: 177–191
http://dx.doi.org/10.1016/j.cell.2008.01.047
Theologis A., Oeller P.W., Wong L.M., Rottmann W.H., and Gantz D.M., 1993, Use of a tomato mutant constructed with reverse genetics to study fruit ripening, a complex developmental process, Developmental Genetics, 14: 282-295
http://dx.doi.org/10.1002/dvg.1020140406
Thomma B.P., Eggermont K., Tierens K.F., and Broekaert W.F., 1999, Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol., 121: 1093–1102
http://dx.doi.org/10.1104/pp.121.4.1093
Tornero P., Gadea J., Conejero V., and Vera P., 1997, Two PR-1 genes from tomato are differentially regulated and reveal a novel mode of expression for a pathogenesis-related gene during the hypersensitive response and development, Mol Plant-Microbe Interact, 10: 624–634
http://dx.doi.org/10.1094/MPMI.1997.10.5.624
Tsuchisaka A., and Theologis A., 2004, Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1- carboxylate synthase gene family members, Plant Physiol, 136: 2982–3000
http://dx.doi.org/10.1104/pp.104.049999
Tuteja N., 2007, Abscisic acid and abiotic stress signaling, Plant Signaling, Behav, 2: 135-8
http://dx.doi.org/10.4161/psb.2.3.4156
Wang S., Tiwari S.B., Hagen G., and Guilfoyle T.J., 2005, Auxin response factor7 restores the expression of auxin-responsive genes in mutant Arabidopsis leaf mesophyll protoplasts, Plant Cell, 17: 1979–1993
http://dx.doi.org/10.1105/tpc.105.031096
Yang S.F., and Hoffman N.E., 1984, Ethylene biosynthesis and its regulation in higher plants, Annu. Rev. Plant Physiol., 35: 155–89
http://dx.doi.org/10.1146/annurev.pp.35.060184.001103
Zarembinski T.I., and Theologis A., 1994, Ethylene biosynthesis and action: a case of conservation, Plant Mol. Biol., 26: 1579–97
http://dx.doi.org/10.1007/BF00016491
Zegzouti H., Jones B., Frasse P., Marty C., Maitre B., Latche A., Pech J.C., and Bouzayen M., 1999, Ethylene-regulated gene expression in tomato fruit: characterization of novel ethylene responsive and ripening-related genes isolated by differential display, The Plant Journal, 18: 589-600
. PDF(328KB)
. FPDF
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Siddra Ijaz
Related articles
. Ethylen
. Biosynthetic pathways
. Ethylene signaling
. Receptor
. Plant physiology
. Growth hormones
Tools
. Email to a friend
. Post a comment
.png)
.png)


