Research Article
Effect of Epichloë gansuensis on Nutrient Resorption Efficiency, Metal Ion Concentrations and Nutrient Ratios of Achnatherum inebrians under Various Nitrogen Concentrations 
2 State Key Laboratory of Plateau Ecology and Agriculture, Qinghai University, Xining 810016, China
3 Retired Scientist of AgResearch, Grasslands Research Centre, Private Bag 11-008, Palmerston North 4442, New Zealand
 Author
Author  Correspondence author
Correspondence author
Molecular Plant Breeding, 2021, Vol. 12, No. 32 doi: 10.5376/mpb.2021.12.0032
Received: 25 Aug., 2021 Accepted: 14 Sep., 2021 Published: 20 Oct., 2021
Cheng C., Liu R.G., Hou W.P., Christensen M.J., Liu Y.L., and Wang J.F., 2021, Effect of Epichloë gansuensis on nutrient resorption efficiency, metal ion concentrations and nutrient ratios of Achnatherum inebrians under various nitrogen concentrations, Molecular Plant Breeding, 12(32): 1-12 (doi: 10.5376/mpb.2021.12.0032)
It is well known that low N limits crop yield, but plants can rationally use nutrients to improve the plant growth at adverse environments. However, the effects of E. gansuensis on nutrient resorption efficiency and metal ion contents of Achnatherum inebrians at low N were unknown. A. inebrians with (E+) and without E. gansuensis (E-) were treated with 0.1 mM (low) N and 7.5 mM (normal) N, after 90 days, the dry weight, the contents of N, P, K, Na, Mg, Zn, Cu, Ca and Fe in senescent and green leaves, N/P/K resorption efficiency at 0.1 mM N and 7.5 mM N were assayed. We found that E. gansuensis enhanced the dry weight of green leaves of A. inebrians, and increased nitrogen resorption efficiency (NRE) of host plant, and the content of N and P in green leaves at 0.1 mM N, but decreased N content in senescent leaves compared to E- plant at 0.1 mM N. The E. gansuensis played an important role in improving host grasses growth at low-N stress by enhancing NRE, and influencing the content of Na, Mg, Zn, Cu, Ca and Fe in senescent leaves and green leaves.
Introduction
Nitrogen (N) is one of the indispensable elements in the whole life cycle of plants, and nitrogen utilization seriously impacts on growth condition and productivity of plants whether in natural or agricultural environment (Xu et al., 2012). There are two beneficial strategies that can be formulated by plants to cope with the deficiencies of nutrition: enhancing nutrient absorption and facilitating nutrient conservation (Aerts and Chapin, 1999). Nutrient absorption is the most essential mechanism for plants to conserve nutrient, which allows plants to make direct re-use of nutrients to adapt to environmental stress (Aerts and Chapin, 1999; Lü et al., 2012). Through this process, plants can transfer a large amount of nutrients from senescent tissues to fresh tissues or other needed tissues (Tully et al., 2013). The formation of nutrient efficiency in plants is a complex process, the percentage of nutrient transport from senescent leaves to green leaves is indicated by nutrient absorption efficiency. Further, NRE (Nitrogen resorption efficiency) and PRE (Phosphorus resorption efficiency) have global averages of ~65 %, and potassium (K) resorption efficiency has global averages of ~70% (Vergutz et al., 2012).
Some studies had reported that increase in nutrient absorption and more effective conservation could change the plant nutrient resorption and their efficiency. Some researchers also reported that the nutrient resorption efficiency differs among habitats (Vergutz et al., 2012), plant species (Diehl et al., 2003; Cai and Bongers, 2007), plant age (Wang et al., 2014), developmental stage (Brant and Chen, 2015), and is easily affected by water and nitrogen content of soil (Lü and Han, 2010). Generally, the process by which plants obtain nutrients from soil is balanced with the process of resorption (Vergutz et al., 2012). The resorption efficiency and resorption proficiency are two merits for the re-use of plants during the process of nutrient resorption (Aerts, 1996). The study reported that a strong resorption is vital for the plant in conditions of nutrient deficiency. Most studies have shown that the relationship about the availability of nutrient and nutrient resorption efficiency is multivariable, and the relationship can be negative (Li et al., 2010b; Vergutz et al., 2012), positive (Yuan et al., 2005) or uninfluenced (Aerts and Chapin, 1999; Yuan and Chen, 2009). One study showed that the addition of N reduced NRE but the change of PRE was not obvious (Lü et al., 2011). Another study had shown that N addition influenced plants PRE (Van Heerwaarden et al., 2003). The nutrients resorption process has a strong link with plant element stoichiometry, since plant growth depends on the availability of nutrients as well as the balance of multiple elements (Ågren, 2008). The resorption can’t regulate the cycling of individual elements but affects multiple elements (Wang et al., 2014). Thus, multiple elements measurement may shed more light on the relationship between RE and elements concentrations rather than individual elements measurement. Meanwhile, plants require mineral elements for normal growth and development, including copper (Cu), calcium (Ca), zinc (Zn), magnesium (Mg), manganese (Mn), iron (Fe), sodium (Na) (Marschner, 2012), and these elements are closely related with crop yield and plant growth, and one element deficiency was usually influenced the other elements metabolism (Liu et al., 2009), for example, improvement of N status would enhance the uptake, remobilization and translocation of Zn in wheat (Erenoglu et al., 2011). The low N probably has influence in mineral balances in plants, further, indirectly or directly regulates mineral contents (Schreiner et al., 2013). Some researchers have indicated that P deficiency increased accumulation of Ca, Fe, Al and Zn, but reduced K and N content (Li et al., 2010a).
Grasses of the sub-family Pooideae are host to endophyte of the genus Epichloë. Endophyte enables the absorption and utilization of plants, and thus thrive in soils low in nutrients (Rahman and Saiga, 2005). Achnatherum inebrians is an invasive grass, which widely distributed in the arid and semi-arid grasslands of northwest China. The feature of this grass is that nearly every plant is infected with a systemic seed-borne fungal endophyte (Chen et al., 2000). Studies have shown that these epichloid endophytes can enhance the resistance of host grasses which are under biotic and abiotic stresses, for instance, it can enhance salt tolerance (Song et al., 2015; Wang et al., 2020), low nitrogen tolerance (Wang et al., 2018a; Wang et al., 2018b; Wang et al., 2020), and Epichloë endophyte influence soil enzymes activity and soil nutrients content in A. inebrians field at the different growth seasons (Hou et al., 2020). However, no research has investigated that the effects of E. gansuensis on the NRE, PRE and KRE, and the content of N, P, K, Na, Mg, Zn, Cu, Ca and Fe in green and senescent leaves of A. inebrians under different N concentrations. Therefore, our study aims would be to test: 1) To determine the concervation of N, P and K in green and senescent leaves, and NRE, PRE and KRE in E+ and E- A. inebrians at different concentrations of N; 2) To determine the ratios of N:P, N:K and P:K, and the contents of Na, Mg, Zn, Cu, Ca and Fe in green and senescent leaves of E+ and E- plants at different N concentrations.
1 Materials and Methods
1.1 Plant growth conditions and nitrogen treatments
E+ and E- A. inebrians seedlings were prepared and treated with the different N concentration as description in our previous study with some modifications (Wang et al., 2018a; Wang et al., 2018b). In short, we used E+ seeds and E- seeds were all originated from a single E+ A. inebrians plant, and half seeds from the single E+ plants were treated with 100 times dilution thiophanate methyl for 2 h, and rinsed the treated seeds with sterile water after that, which was a useful way to kill the endophyte of A. inebrians (Hou et al., 2020), these treated seeds were sowed in the field, and harvested E- seeds, and these E- seeds and the other half seeds of the single E+ plants were used in this study. Sowed 6 healthy and full A.inebrians seeds in 12 pots (E+ plants) and 12 pots (E- plants), respectively. After entering the germination state, each pot (lower diameter (10 cm) × upper diameter (18.5 cm) × height (19.5 cm) kept three seedlings, and prepared two sets of trays for the pots, each tray contains 6 pots. Irrigated all the trays on a seven-day cycle. After the second leaf of A. inebrians seedlings appeared and totally expanded, 1/2 concentration of Hoagland's solution was applied to the pots every 7 days. The greenhouse where these trays were located is constant temperature (temperature: (26±2)°C, moisture: (42±2)%). After 45 days, the modified 1/2 Hoagland's solution containing 0.1 mM N and 7.5 mM N was used to treat 12 pots of E- plants and 12 pots of E+ plants for a further 90 days, and 150 mL of two different N concentrations was applied every 7 days to the corresponding treatment pots. Through the aniline blue method, the seedlings infection status was confirmed. Both E+ and E- plants were applied to each treatment, and each N treatment included six replications.
1.2 The dry weight of green and senescent leaves
After 90 days of treatment, all E+ and E- plants were carefully removed from the vermiculite and the green and senescent leaves were manually separated. To determine the dry weights, green leaves and senescent leaves of each pot of every N treatment were dried in an oven at 80°C until them reached a constant weight.
1.3 Determination of N, P and K content
Flow injection analysis was used to assay the total N and P content in green and senescent leaves (FIAstar 5000 Analyzer, FOSS, Sweden), the method was carried out by the description of Wang et al. (2018b). K content in green leaves and senescent leaves was determined by digital flame analyzer (2655-00, Chicago, USA), the method was performed by the description of Bao et al. (2016). The content of Na, Mg, Zn, Cu, Ca and Fe in green leaves and senescent leaves were analyzed with ICP MS (ICAP RQ), using HF, HNO3and HCl for digestion for 8 min in 120°C, and then 8 min in 150°C, finally, 30 min in 180°C, volume to 25 mL.
1.4 Calculation of nutrient RE and stoichiometric ratios
The nutrient RE of A. inebrians was calculated with the following formula on the basis of mass (Kobe et al., 2005; Huang et al., 2007): Nutrient RE = 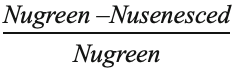 ×100%, where Nugreen means the nutrient content in the green leaves and Nusenescent means the nutrient content in the senescent leaves. E+ and E- ratios of N, P and K in green leaves and senescent leaves of plants were calculated based on N:P, N:K, and P:K.
×100%, where Nugreen means the nutrient content in the green leaves and Nusenescent means the nutrient content in the senescent leaves. E+ and E- ratios of N, P and K in green leaves and senescent leaves of plants were calculated based on N:P, N:K, and P:K.
1.5 Statistical analysis
Under the same N concentration, independent T-test was used to carry out the significance of difference for the all parameters of E+ and E- plants, and the significance of difference between green leaves and senescent leaves for all parameters was conducted by independent T-tests under the same N concentration. The statistical significance was defined at the 95% confidence level. Mean value are expressed as its standard errors. Principal component analysis was performed with the content of N, P, K, Na, Mg, Zn, Cu, Ca and Fe by R. The two-way ANOVA analyzed the effects of endophyte (E) and N concentration (NC) on NRE, PRE and KRE, the three-way ANOVA analyzed the effects of endophyte (E), nitrogen concentration (NC), and tissues (T) on the contents of N, P, K, Na, Mg, Zn, Cu, Ca and Fe, and the ratios of N:P, N:K and P:K.
2 Results
2.1 Dry weights of green and senescent lamina of E+ and E- plants
After 90 days of treatment with two concentrations of external nitrogen, our results showed that the dry weight of E+ and E- green leaves of under 0.1 mM N treatment was significantly reduced compared to 7.5 mM N (Figure 1), but the present of E. gansuensisincreased the dry weight of green leaves compared to E- green leaves under 0.1 mM N. Further, low-N stress significantly increased the dry matter of senescent leaves in E+ and E- plants compared to the 7.5 mM N (Figure 1). The dry weight of senescent leaves had no significant differences between E+ and E- plants under 0.1 mM N and 7.5 mM N, respectively. The dry weight of green leaves was higher than senescent leaves in E+ and E- plants under 7.5 mM N, however, the dry weight of senescent leaves was lower than green leaves in E+ plants under low N condition.
|
Figure 1 The dry weight of the green leaf and senescent leaf in E+ and E- plants at the various N concentration Note: The asterisk above the bar chart represents significant difference between green leaf and senescent leaf at the same N concentration. The green asteriskindicates significant difference between the green leaf of E+ and the green leaf of E- at 0.1 mM N and 7.5 mM N, respectively. The blue asterisk indicatessignificant difference between the senescent leaf of E+ and the senescent leaf of E- at 0.1 mM N and 7.5 mM N, respectively. *, ** and *** means significant difference at P<0.05, P<0.01 and P<0.001, and n means no significant difference |
2.2 The effect of E. gansuensis on the content of N, P and K of the green and senescent leaves under the different N concentration
The N, P and K content of green leaves was higher than senescent leaves in E+ and E- plants under two N concentrations, respectively. Further, our results indicated that 0.1 mM N decreased the content of N, P and K of E+ and E- plants compared with the 7.5 mM N (Figure 2). However, the N content of green and senescent leaves, and P content of green leaves had obvious differences between E+ and E- plants under 0.1 mM N (Figure 2a; Figure 2b). The N and P content of the green leaves of E- plants were lower than the counterparts of E+ plants under low N condition, and N and P content of the green leaves of E+ plants were enhanced by 19.9% and 12.7% compared with the green leaves of E- plants under 0.1 mM N, respectively (Figure 2a). The N content of the senescent leaves in E+ plants was lower than the counterparts of E- plants under 0.1 mM N (Figure 2a), and the N content of the senescent leaves of E+ plants was decreased by 11.9% compared with the senescent leaves of E- plants under 0.1 mM N (Figure 2a). There was no obvious difference in K content in green leaves of E+ and E- plants under 0.1 mM N and 7.5 mM N, the result was consistent with the K content of senescent leaves (Figure 2c). As shown in Table 1, the endophyte-infection did not cause a remarkable effect on the content of N, P and K, while nitrogen concentration (NC) and the tissues (T) caused marked effects on the content change of N, P and K, respectively (P<0.001). In addition, the interaction of NC × T had clear differences on the content of N (P<0.001) and K (P=0.016).
|
Figure 2 The N content (a), P content (b) and K content (c) of the green and senescent leaves of E+ and E- plants at the different N concentration Note: The asterisk above the bar chart indicates significant difference between green leaf and senescent leaf at the same N concentration. The green asteriskindicates significant difference between the green leaf of E+ and the green leaf of E- at 0.1 mM N and 7.5 mM N, respectively. The blue asterisk indicatessignificant difference between the senescent leaf of E+ and the senescent leaf of E- at 0.1 mM N and 7.5 mM N, respectively. *, ** and *** means significant difference at P<0.05, P<0.01 and P<0.001, and n means no significant difference |
|
Table 1 Results of three-way ANOVA for the effects of endophyte (E), nitrogen concentration (NC) and tissues (T) on the content of N, P and K, and the ratios of N:P, N:K and P:K |
2.3 The effect of E. gansuensis on NRE, PRE and KRE of A. inebrians at the various N concentrations
The presence of E. gansuensis increased NRE compared to E- plants under 0.1 mM N, and NRE of E+ plants were enhanced by 27.4 % compared with those of E- plants under 0.1 mM N (Figure 3a). However, NPE PRE and KRE had not affected by endophyte, under normal N condition (Figure 3). The PRE and KRE had no obvious differences between E+ plants and E- plants under 0.1 mM N (Figure 3b; Figure 3c). NC caused obvious effects on the NRE (P=0.049) and PRE (P<0.001) (Table 2).
|
Figure 3 Resorption efficiencies of nitrogen (a), phosphorus (b) and potassium (c) of E+ and E- plants under the different N concentration Note: The asterisk represents significant difference between E+ and E- plants at the same N concentration. * represents significant difference at P<0.05 |
|
Table 2 Results of two-way ANOVA for the effects of endophyte (E) and nitrogen concentration (NC) on NRE, PRE and KRE |
2.4 The ratios of N:P, N:K and P:K of the green leaves and senescent leaves in E+ and E- plants
The content of N, P and K of the green and senescent leaves were distinct, thus their ratios also varied differently (Figure 4). Our research shown that 0.1 mM N decreased the ratios of N:P and N:K of the green leaves compared with 7.5 mM N in E+ plants and E- plants (Figure 4a; Figure 4b), and 0.1 mM N decreased P:K ratio of the senescent leaves compared with 7.5 mM N in E+ plants and E- plants (Figure 4c). The N:P ratio of the green leaves and the N:K ratio of the green leaves had clear differences between E+ and E- plants, respectively, under 0.1 mM N (Figure 4a; Figure 4b), with the N:P ratio of the green leaves in E+ plants increased by 8.49% compared with the green leaves of E- plants. The data also had shown that the N:K ratio of the green leaves in E+ plants was decreased by 22.65% compared with the counterpart of E- plants under 0.1 mM N (Figure 4b). Furthermore, Table 1 showed that the endophyte did not have effect on the ratios of N:P, N:K and P:K, while NC caused the notable effects on the ratios of N:P (P<0.001) and N:K (P<0.001), and T also caused the marked effects on the ratios of N:P (P<0.001), N:K (P<0.001) and P:K (P<0.001). In addition, the interaction of NC × T had clear differences on the ratio of N:P (P<0.001), N:K (P<0.001) and P:K (P=0.003).
|
Figure 4 The N:P (a), N:K (b) and K:P (c) of the green and senescent leaves of E+ plants and E- plants under the different N concentration between E+ and E- plants Note: The asterisk above the bar chart represents significant difference between green leaf and senescent leaf at the same N concentration. The dark yellow asterisk means significant difference between the green leaf of E+ and the green leaf of E- at 0.1 mM N and 7.5 mM N, respectively. The purple asterisk represents significant difference between the senescent leaf of E+ and the senescent leaf of E- at 0.1 mM N and 7.5 mM N, respectively. *, ** and *** means significant difference at P<0.05, P<0.01 and P<0.001, and n means no significant difference |
2.5 The effect of E. gansuensis on the content of metal ion in green leaves and senescent leaves under the various N concentrations
The content of Na, Mg and Ca of the green leaves was dramatically lower than the senescent leaves in E+ and E- plants under the two N concentrations, respectively (Figure 5a; Figure 5b; Figure 5e). We also found that the contents of Zn and Cu of the green leaves were both significantly higher than the senescent leaves in E+ and E- plants under low N treatment (Figure 5c; Figure 5d). Under 7.5 mM N, the Fe content of the green leaves was remarkably lower than the senescent leaves in E+ and E- plants (Figure 5f). Further, E. gansuensismarkedly reduced the Zn content of green leaves compared with the counterpart of E- plants under 0.1 mM N (Figure 5c), but E. gansuensis clearly increased the content of Na, Mg, Zn, Cu, Ca and Fe of green leaves compared with the counterpart of E- plants under 7.5 mM N. E. gansuensis significantly decreased the content of Na, Mg, Zn and Ca of senescent leaves compared with the counterpart of E- plants under 7.5 mM N, however, E. gansuensis enhanced the content of Na, Mg, Zn and Ca of senescent leaves compared with the counterpart of E- plants under low N treatment (Figure 5). Table 3 demonstrated that endophyte had significant effect on the content of Cu (P<0.001), Ca (P<0.001) and Fe (P<0.001), while NC caused clear effects on the content of Na (P<0.001), Mg (P<0.001), Zn (P<0.001) and Cu (P<0.001). In addition, the interaction of E × NC × T had clear differences on the content of Na (P<0.001), Mg (P<0.001), Zn (P<0.001), Cu (P=0.028) and Ca (P<0.001).
|
Figure 5 The Na content (a), Mg content (b), Zn content (c), Cu content (d), Ca content (e) and Fe content (f) of the green and senescent leaves of E+ and E- plants under different N concentration treatments Note: The asterisk above the bar chart represents significant difference between green leaf and senescent leaf at the same N concentration. The green asteriskmeans significant difference between the green leaf of E+ and the green leaf of E- at 0.1 mM N and 7.5 mM N, respectively. The blue asterisk indicates that the significant difference between the senescent leaf of E + and those of E- is 0.1 mM N and 7.5 mM N, respectively. *, ** and *** means significant difference at P<0.05, P<0.01, P<0.001, respectively, and n means no significant difference |
|
Table 3 Results of three-way ANOVA for the effects of endophyte (E), nitrogen concentration (NC) and tissues (T) on the content of Na, Mg, Zn, Cu, Ca and Fe |
2.6 Principal component analysis
Some variables represented distinct separation, and the factor 1 could explain 50.39% of the total variance in green leaves, on which Cu content contributed the most variation (45.7%), while Mg content and Zn content accounted for approximately 44.7% and 41.5%, respectively (Figure 6). N content (50.9%) and K content (46.2%) were loaded on factor 2, which could explain 39.15% of the variation. The difference between 0.1mM and 7.5 mM N from E+ and E- plants could be seen in the PCA plot (Figure 6). However, distinct separation of some variables was showed, and in senescent leaves, 68.48% of the total variance was explained by factor 1, on which Mg content contributed the most variation (37.0%), while P and Zn content accounted for approximately 36.6% and 36.0%, respectively (Figure 7). Cu content (52.5%) and Na content (48.5%) were loaded on factor 2, and which could explain 20.98% of the variation. The differences between 0.1 mM N and 7.5 mM N from E+ plants and E- plants were shown by PCA plot (Figure 7).
|
Figure 6 Principal component analysis for endophyte-infection and N treatment with the content of N, P, K, Na, Mg, Zn, Cu, Ca and Fe in green leaf |
|
Figure 7 Principal component analysis for endophyte-infection and N treatment with the content of N, P, K, Na, Mg, Zn, Cu, Ca and Fe in senescent leaf |
3 Discussion
Based on our research results, the E. gansuensis enhanced the adaptation of the host grass to low N stress, and it included the dry weight of green leaves in E+ plants were higher than the counterpart of E- plants. However, the dry weight of senescent leaves was not affected by endophyte when the plant suffers from low nitrogen stress. Some studies also showed that the E. gansuensis had powerful biological functions and improved A. inebrians resistance to abiotic stresses, and increased dry weight of host grasses compared to E- plants (Wang et al., 2018a; Wang et al., 2018b), therefore, our present results were coincident with the previous results.
Some previous studies had reported that with the nitrogen supply increased, the NRE of some ecosystems remained unchanged or decreased (Van Heerwaarden et al., 2003; Güsewell, 2005b; Kozovits et al., 2007) However, the other study indicated that the addition of N caused plants to show a specific PRE response (Van Heerwaarden et al., 2003). One study concluded that the resorption efficiency of plant specific nutrient might not have much to do with nutrient status (Aerts, 1996). Additionally, the degree of senescence might partly contribute to the low RE, and elements also might be through the production and decomposition of leaf litter back into the soil. However, some of the elements from senescent leaves were translocated to other tissues of plants in the later stages of senescence (Blanco et al., 2009; Lü et al., 2012). There were no study reports that the roles of E. gansuensis endophyte on nutrient resorption efficiency of host grasses under different N concentration. Current research shown that, both NRE of E- plant and KRE of E+ plant was affected by N supply. Our results suggested that compared with E- plants, E. gansuensis increased host plants NRE under 0.1 mM N stress, which indicated more N was transferred from the senescent leaves and reused in green leaves of E+ plants under 0.1 mM N condition; however, the infection of E. gansuensis did not affect the PRE and KRE of host grasses under the two different N treatments. Improvement of N utilization efficiency in the plants will enhance plant growth under low N stress.
N, P and K are very important elements for plant growth (Amtmann and Rubio, 2012), and as the plant grows, the contents of N, P and K in leaves are constantly changing (Alam et al., 2011). The balance of nutrients can influence plant growth rates, and the N, P and K ratio in ecosystems has been widely studied (Wang et al., 2014; Scalon et al., 2017). In the current study, we investigated different responses of E+ and E- A. inebrians to low N conditions and analyzed the possible changes of C, N and P content in green and senescent leaves. Few studies have revealed the effect of endophyte on difference of N concentration from the perspective of N, P, K ratio. In this study, there were effects of E. gansuensis on the N content of green and senescent leaves and the P content of green leaves under 0.1 mM N. Further, our study indicated that the presence of E. gansuensis increased N and P content of the green leaves but decreased the N content of the senescent leaves under 0.1 mM N. Recently, it was reported that an epichloid endophyte increased the P and N content in Hordeum brevisubulatum plants under NaCl stress (Song et al., 2015; Chen et al., 2018). A previous study had shown that E. coenophila(Neotyphodium coenophialum) affected mineral absorption, transportation and nutrient ratios of tall fescue (Festuca arundinacea) (Rahman and Saiga, 2005). Similarly, E. festucae changed the nutrient composition of F. rubra, and the content of P (62%) and N (19%) in E+leaves were more than E- plants (Vázquez-de-Aldana et al., 2013). This study had provided evidence that the existence of epichloid endophytes in grasses can affect the availability of nutrients to growing leaves of host grasses through enhanced resorption from senescent leaves to improve host adaptability to environment conditions. All in all, the existence of E. gansuensis increased the NRE of A. inebrians to improve host grasses growth under N deficiency stress, and to enhance the nutrient use efficiency in the case of limited nutrients. Due to changes of N, P and K content, there was only change for the N:P ratio of the green leaves under 0.1 mM N.
There are conclusions about the relationships between plant resorption process and nutrition status (Diehl et al., 2003; Kobe et al., 2005; Vergutz et al., 2012), for example, according to the reports, in mature leaf nitrogen content was positively correlated with the absorption of nitrogen in forests. (Diehl et al., 2003). However, it was found that an increase in nutritional status was globally associated with a decrease in NRE, PRE and KRE (Kobe et al., 2005; Vergutz et al., 2012). Meanwhile, several studies have reported that N and P interact functionally, NRE and PRE are tightly coupled (Güsewell, 2005a; Ågren, 2008). Although plants growing in poor environment always had longer leaf life and lower nutrient concentration, the negative correlation between nutrient availability and NRE has not proven to be a consistent property (Kobe et al., 2005). Low-N stress and E. gansuensis also influenced the related elements of photosynthesis, for example, the net photosynthetic rate, chlorophyll a and chlorophyll b (Wang et al., 2018a). Fe is also related with the ability of carbon assimilation, and it plays an essential role in the chlorophyll synthesis in photosynthesis. The results come from our research showed that E. gansuensis enhanced the green leaves Fe content under 7.5 mM N, and Fe is a coenzyme factor of NR (nitrate reductase), NiR (nitrite reductase) and GS (glutamate synthase) in the pathway of N metabolism (Borlotti et al., 2012), interestingly, E. gansuensis increased the activity of NR, NiR and GS under 0.1 mM N (Wang et al., 2018b). Zn is also important element for the synthesis of chlorophyll. In E+ plants, N deficiency reduced Zn content of green leaves, but low N improved Zn content of green leaves of. E. gansuensis probably play an essential role in regulating the balance of iron to improve host grasses growth under the environment stress.
In nutrient-poor environments, the efficiency of nutrient recycling affects plants growth and reproduction (May and Killingbeck, 1992). Plants vary greatly in the efficiency with which nutrients are resorbed before leaf abscission. In our study, there was remarkable positive correlation between dry weight of green leaves and KRE in E+ plants, and there was also significant positive correlation between the dry weight of senescent leaves and PRE in E+ plants. e However, in E- plants, there was no obvious relationship between dry weight of leaves and REs. Therefore, it seems to be generally true that the presence of the endophyte affected the correlation between leaves dry weight and REs.
In summary, E. gansuensis played an important role on increasing NRE, the content N and P of green leaves under 0.1 mM N, and influencing the content of Na, Mg, Zn, Cu, Ca and Fe of the green leaves and senescent leaves to improve A. inebrians growth under low-N stress. The present study highlight that it is meaningful to investigate the responses of host plants to E. gansuensis, not only to elucidate the role of endophyte but also to understand the nutrients resorption from the senescent leaves to green leaves. Our results had expanded the biological functions of E. gansuensis endophyte, and E. gansuensican be used to cultivate new forage germplasm with high nutrient re-absorption efficiency in the future.
Author contributions
CC, WJF conceived and designed the research. CC, WJF, LRG, HWP performed the research. CC, LYL, WJF participated in the experiments and analyzed data. CC, WJF, MJC drafted and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This research was financially supported by Program for Changjiang Scholars and Innovative Research Team in University (IRT_17R50), the Joint Fund of the Natural Science Foundation of China and the Karst Science Research Center of Guizhou Province (Grant No. U1812401), the Natural Science Foundation of China (32001399, 32001387, 31971768), the Technical Service Agreement for Research and Development of Beneficial Microbial Agents of Rhododendron Alpine (071200001), Lanzhou University “Double First-Class” guiding special project-team construction fund-scientific research start-up fee standard (561119206), the Fundamental Research Funds for the Central Universities (lzujbky-2021-ey01, lzujbky-2021-kb12) in Lanzhou University, the Open Project of State Key Laboratory of Plateau Ecology and Agriculture, Qinghai University (2021-KF-02).
Competing interests
The authors declare that they have no competing interests
Aerts R., 1996, Nutrient resorption from senescing leaves of perennials: are there general patterns? J. Ecol., 84: 597-608
https://doi.org/10.2307/2261481
Aerts R., and Chapin F.S., 1999, The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns, Adv. Ecol. Res., 30: 1-67
https://doi.org/10.1016/S0065-2504(08)60016-1
Ågren G.I., 2008, Stoichiometry and nutrition of plant growth in natural communities, Annu. Rev. Ecol. Evol. Syst., 39: 153-170
https://doi.org/10.1146/annurev.ecolsys.39.110707.173515
Alam R., Iqbal A., Khan I., Ali I., Munir I., Tahir M., Jan N., Swati A., 2011, Carbon and nitrogen stoichiometry in Brassica napus L. seedlings after supplementation with Ca2+ and K+ under irrigated and drought stress conditions, Afr. J. Biotechnol., 10: 18418-18424
https://doi.org/10.5897/AJB11.1407
Amtmann A., and Rubio F., 2012, Potassium in plants, eLS, pp. 1-10
https://doi.org/10.1002/9780470015902.a0023737
Bao A.K., Du B.Q., Touil L., Kang P., Wang Q.L., Wang S.M., 2016, Co-expression of tonoplast Cation/H+ antiporter and H+-pyrophosphatase from xerophyte Zygophyllum xanthoxylumimproves alfalfa plant growth under salinity, drought and field conditions, Plant Biotechnol. J., 14(3): 964-975
https://doi.org/10.1111/pbi.12451
PMid:26268400
Blanco J.A., Imbert J.B., Castillo F.J., 2009, Thinning affects nutrient resorption and nutrient‐use efficiency in two Pinus sylvestris stands in the Pyrenees, Ecol. Appl., 19: 682-698
https://doi.org/10.1890/1051-0761-19.3.682
PMid:19425431
Borlotti A., Vigani G., Zocchi G., 2012, Iron deficiency affects nitrogen metabolism in cucumber (Cucumis sativus L.) plants, BMC Plant Biol., 12: 1
https://doi.org/10.1186/1471-2229-12-189
PMid:23057967 PMCid:PMC3539955
Brant A.N., Chen H.Y., 2015, Patterns and mechanisms of nutrient resorption in plants, Crit. Rev. Plant. Sci., 34: 471-486
https://doi.org/10.1080/07352689.2015.1078611
Cai Z.Q., and Bongers F., 2007, Contrasting nitrogen and phosphorus resorption efficiencies in trees and lianas from a tropical montane rain forest in Xishuangbanna, south-west China, J. Trop. Ecol., 23: 115-118
https://doi.org/10.1017/S0266467406003750
Chen, T., Johnson, R., Chen, S., Lv, H., Zhou, J., Li, C., 2018, Infection by the fungal endophyte Epichloë bromicola enhances the tolerance of wild barley (Hordeum brevisubulatum) to salt and alkali stresses, Plant Soil, 428 (1-2):353-370
https://doi.org/10.1007/s11104-018-3643-4
Chen L., Li X., Li C., Swoboda G.A., Young C.A., Sugawara K., Leuchtmann A., Schardl C.L., 2015, Two distinct Epichloë species symbiotic with Achnatherum inebrians, drunken horse grass. Mycologia, 107: 863-873
https://doi.org/10.3852/15-019
PMid:25911697
Christensen M.J., Bennett R.J., Ansari H.A., Koga H., Johnson R.D., Bryan G.T., Simpson W.R., Koolaard J.P., Nickless E.M., Voisey C.R., 2008, Epichloë endophytes grow by intercalary hyphal extension in elongating grass leaves, Fungal Genet. Biol., 45: 84-93Diehl P., Mazzarino M., Funes F., Fontenla S., Gobbi M., Ferrari J., 2003, Nutrient conservation strategies in native Andean-Patagonian forests, J. Veg. Sci., 14: 63-70
https://doi.org/10.1016/j.fgb.2007.07.013
PMid:17919950
Erenoglu E.B., Kutman U.B., Ceylan Y., Yildiz B., Cakmak I., 2011, Improved nitrogen nutrition enhances root uptake, root-to-shoot translocation and remobilization of zinc (65Zn) in wheat, New Phytol., 189: 438-448
https://doi.org/10.1111/j.1469-8137.2010.03488.x
PMid:21029104
Güsewell S., 2005a, High nitrogen: phosphorus ratios reduce nutrient retention and second-year growth of wetland sedges, New Phytol., 166: 537-550
https://doi.org/10.1111/j.1469-8137.2005.01320.x
PMid:15819916
Güsewell S. 2005b, Nutrient resorption of wetland graminoids is related to the type of nutrient limitation, Funct. Ecol., 19: 344-354
https://doi.org/10.1111/j.0269-8463.2005.00967.x
Hou W., Wang J., Nan Z., Christensen M.J., Xia C., Chen T., Zhang Z., Niu X., 2020, Epichloë gansuensis endophyte-infection alters soil enzymes activity and soil nutrients at different growth stages of Achnatherum inebrians, Plant Soil, 455: 227-240
https://doi.org/10.1007/s11104-020-04682-2
Huang J., and Boerner R., 2007, Effects of fire alone or combined with thinning on tissue nutrient concentrations and nutrient resorption in Desmodium nudiflorum, Oecologia, 153(2):233-243
https://doi.org/10.1007/s00442-007-0733-z
PMid:17453253
Kobe R.K., Lepczyk C.A., Iyer M., 2005, Resorption efficiency decreases with increasing green leaf nutrients in a global data set, Ecology, 86: 2780-2792
https://doi.org/10.1890/04-1830
Kozovits A., Bustamante M., Garofalo C., Bucci S., Franco A., Goldstein G., Meinzer F., 2007, Nutrient resorption and patterns of litter production and decomposition in a Neotropical Savanna, Funct. Ecol., 21: 1034-1043
https://doi.org/10.1111/j.1365-2435.2007.01325.x
Lü X.T., Cui Q., Wang Q.B., Han X.G., 2011, Nutrient resorption response to fire and nitrogen addition in a semi-arid grassland, Ecol. Eng., 37: 534-538
https://doi.org/10.1016/j.ecoleng.2010.12.013
Lü X.T., and Han X.G., 2010, Nutrient resorption responses to water and nitrogen amendment in semi-arid grassland of Inner Mongolia, China, Plant Soil, 327: 481-491
https://doi.org/10.1007/s11104-009-0078-y
Lü X.T., Freschet G.T., Flynn D.F., Han X.G., 2012, Plasticity in leaf and stem nutrient resorption proficiency potentially reinforces plant-soil feedbacks and microscale heterogeneity in a semi‐arid grassland, J. Ecol., 100: 144-150
https://doi.org/10.1111/j.1365-2745.2011.01881.x
Li L., Liu C., Lian X., 2010a, Gene expression profiles in rice roots under low phosphorus stress, Plant Mol. Biol., 72:423-432
https://doi.org/10.1007/s11103-009-9580-0
PMid:19936943
Li X., Zheng X., Han S., Zheng J., Li T., 2010b, Effects of nitrogen additions on nitrogen resorption and use efficiencies and foliar litterfall of six tree species in a mixed birch and poplar forest, northeastern China, J. forest Res., 40: 2256-2261
https://doi.org/10.1139/X10-167
Liu T.Y., Chang C.Y., Chiou T.J., 2009, The long-distance signaling of mineral macronutrients, Curr. Opin. Plant Biol., 12: 312e319
https://doi.org/10.1016/j.pbi.2009.04.004
PMid:19481493
Marschner P., 2012, Marschner's mineral nutrition of higher plants, third ed, Academic Press, London, UK
May J.D., and Killingbeck K.T., 1992, Effects of preventing nutrient resorption on plant fitness and foliar nutrient dynamics, Ecology, 73: 1868-1878
https://doi.org/10.2307/1940038
Rahman M., and Saiga S., 2005, Endophytic fungi (Neotyphodium coenophialum) affect the growth and mineral uptake, transport and efficiency ratios in tall fescue (Festuca arundinacea), Plant Soil, 272: 163-171
https://doi.org/10.1007/s11104-004-4682-6
Scalon M.C., Wright I.J., Franco A.C., 2017, To recycle or steal? Nutrient resorption in Australian and Brazilian mistletoes from three low‐phosphorus sites, Oikos, 126: 32-39
https://doi.org/10.1111/oik.03455
Schreiner R.P., Lee J., Skinkis P.A., 2013, N, P, and K supply to pinot noir grapevines: impact on vine nutrient status, growth, physiology, and yield, Am. J. Enol. Vitic., 64: 26e38
https://doi.org/10.5344/ajev.2012.12064
Song M., Chai Q., Li X., Yao X., Li C., Christensen M.J., Nan Z., 2015, An asexual Epichloë endophyte modifies the nutrient stoichiometry of wild barley (Hordeum brevisubulatum) under salt stress, Plant Soil, 387: 153-165
https://doi.org/10.1007/s11104-014-2289-0
Tully K.L., Wood T.E., Schwantes A.M., Lawrence D., 2013, Soil nutrient availability and reproductive effort drive patterns in nutrient resorption in Pentaclethra macroloba, Ecology, 94: 930-940
https://doi.org/10.1890/12-0781.1
Vázquez-de-Aldana B.R., García-Criado B., Vicente-Tavera S., Zabalgogeazcoa I., 2013, Fungal endophyte (Epichloë festucae) alters the nutrient content of Festuca rubra regardless of water availability, PLoS One, 8: e84539
https://doi.org/10.1371/journal.pone.0084539
PMid:24367672 PMCid:PMC3867530
Van Heerwaarden L., Toet S., Aerts R., 2003, Nitrogen and phosphorus resorption efficiency and proficiency in six sub‐arctic bog species after 4 years of nitrogen fertilization, J. Ecol., 91: 1060-1070
https://doi.org/10.1046/j.1365-2745.2003.00828.x
Vergutz L., Manzoni S., Porporato A., Novais R.F., Jackson R.B., 2012, Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants, Ecol. Monogr., 82: 205-220
https://doi.org/10.1890/11-0416.1
Wang J., Nan Z., Christensen M.J., Li C., 2018a, Glucose-6-phosphate dehydrogenase plays a vital role in Achnatherum inebrians plants host to Epichloë gansuensis by improving growth under nitrogen deficiency, Plant Soil, 430: 37-48
https://doi.org/10.1007/s11104-018-3710-x
Wang J., Nan Z., Christensen M.J., Zhang X., Tian P., Zhang Z., Niu X., Gao P., Chen T., Ma L., 2018b, Effect of Epichloë gansuensis endophyte on the nitrogen metabolism, nitrogen use efficiency, and stoichiometry of Achnatherum inebrians under nitrogen limitation, J. Agr. Food Chem, 66: 4022-31
https://doi.org/10.1021/acs.jafc.0c01396
PMid:32551564
Wang J., Hou W, Christensen M.J., Li X., Xia C., Li C., Nan Z., 2020, Role of Epichloë endophytes in improving host grass resistance ability and soil properties, J. Agr. Food Chem, 68: 6944-6955
Wang Z., Lu J., Yang H., Zhang X., Luo C., Zhao Y., 2014, Resorption of nitrogen, phosphorus and potassium from leaves of lucerne stands of different ages, Plant Soil, 383: 301-312
Xu G., Fan X., Miller A.J., 2012, Plant nitrogen assimilation and use efficiency, Annu Rev Plant Biol., 63: 153-182
https://doi.org/10.1146/annurev-arplant-042811-105532
PMid:22224450
Yuan Z.Y., Li L.H., Han X.G., Huang J.H., Jiang G.M., Wan S.Q., Zhang W.H., Chen Q.S., 2005, Nitrogen resorption from senescing leaves in 28 plant species in a semi-arid region of northern China, J. Arid. Enviro, 63: 191-202
https://doi.org/10.1016/j.jaridenv.2005.01.023
Yuan Z., and Chen H.Y., 2009, Global‐scale patterns of nutrient resorption associated with latitude, temperature and precipitation, Glob. Ecol. Biogeogr., 18: 11-18
. PDF(931KB)
. HTML
Associated material
. Readers' comments
Other articles by authors
. Chen Cheng
. Ronggui Liu
. Wenpeng Hou
. Michael J. Christensen
. Yinglong Liu
. Jianfeng Wang
Related articles
. Epichloë gansuensis
. Low nitrogen
. Nutrient resorption efficiency
. Achnatherum inebrians
Tools
. Email to a friend
. Post a comment
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)



