Effect of Ca2+ Regulators on the CaM Gene Expression in Guzmania ‘Amaranth’ during Flower Induction by Ethylene 
2. Key Laboratory of South China Gene Resources and Germplasm Creating, Ministry of Agriculture, Danzhou, 571737, P.R. China
3. Key Laboratory of Tropical Crops Germplasm Genetic Improvement and Creation of Hainan Province, Danzhou, 571737, P.R. China
4. Vegetable Research Institute, Guangxi Academy of Agricultural Sciences, Nanning, 530007, P.R. China
 Author
Author  Correspondence author
Correspondence author
Plant Gene and Trait, 2012, Vol. 3, No. 3 doi: 10.5376/pgt.2012.03.0003
Received: 11 Nov., 2011 Accepted: 09 Dec., 2011 Published: 01 Mar., 2012
Li et al., 2012, Effect of Ca2+ Regulators on the CaM Gene Expression in Guzmania ‘Amaranth’ during Flower Induction by Ethylene, Plant Gene and Trait, Vol.3, No.3 13-17 (doi: 10.5376/pgt.2012.03.0003)
Since 1930s, the technology of flowering induction by ethylene in bromeliads has begun to be used in production. So far, it is still effectively used in bromeliads flowering induction. However, There is little clear on the mechanism of ethylene flowering induction. In order to clarify the role of Ca2+-CaM system in flowering induction by ethylene in bromeliads, Guzmania ‘Amaranth’ plants were employed to be treated with combination of ethylene and Ca2+ accelerator, or Ca2+ chelator or Ca2+ inhibitor respectively. The expression characteristics of CaM were investigated by the approaches of RT-PCR and Northern blot. The results showed that the Ca2+ accelerator can advance the expression peak of CaM at the same time of ethylence treatment, wheresas, Ca2+ chelator and Ca2+ inhibitor could delay the expression peak of CaM. These results were consistent with the corresponding process of the flowering time, which indicated that the Ca2+-CaM system might play an important regulative role in the flowering induction by ethylene in Guzmania ‘Amaranth’, but not a decisive role.
Bromeliaceae are the monocotyledonous herbaceous plants. Most of them are used as ornamental plants while a few of them are edible. Bromeliads are famous tropical flowers. In order to appear on the market on time, flowering-inducing agents, such as ethylene and its analogue, were used for the cultivation of bromeliads. The rosulated leaf of bromeliads can filtrate the water and nutrition, and the scalelike hairs on the leaf base epidermis can absorb water, nutrition and ethylene (Turnbull et al., 1999). Exogenous ethylene and its analogue might induce bromeliads flower by increasing endogenous ethylene. Treated with inhibitor of endogenous ethylene production and transgenic technology, they can suppress the expression of ACC synzyme gene, which has been proved by many investigators (Burg and Burg, 1966; da Gunha, 2005; Kuan et al., 2005; Dukovski et al., 2006; Wang et al., 2007). However, there is still no specific report on how the endogenous ethylene launches the flower.
Ca2+-CaM has been becoming the hot topic of cytobiology, development biology and phytophysiology. Firstly, Ca2+ is the essential mineral element for plants growth and development. Sencondly, Ca2+ is called the second messenger for its function of coupling the extracellular information and intracellular physiological and biochemical responses. CaM, as the receptor of Ca2+, plays an important role in the signal transduction process and involves in the regulation of many intracellular physiological and biochemical responses through its downstream CaM binding proteins (Mao et al., 2004). Some reports have showed that Ca2+ participated in the plant flower formation (Havelange, 1989; Gong et al., 1990; Poovaiah and Reddy, 1993). However, these are seldom studies directly on the relation between Ca2+-CaM system and flower formation, especially in tropical ornamental plants.
Our earlier studies showed that Ca2+ regulator could regulate the contents of Ca2+ and CaM during ethylene induced flower in bromeliads (Yi et al., 2011). But there were rarely reports about the effects of Ca2+ on the expression of CaM and differentiation of flower buds. In order to further study the probable role of Ca2+-CaM system in bromeliads flower induction with ethylene, we researched the effects of Ca2+ regulators on the expression of CaM and the stage of flower buds development by RT-PCR, Northern blot and anatomy methods. The results might be helpful for controlling flower time of bromeliads and clarifying the mechanism of bromeliads flower.
1 Result and Analysis
1.1 Cloning and sequence analysis of CaM gene fragment
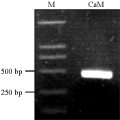
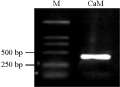
The fragment of PCR was about 444 bp (Figure 1). The size was the same as the fragment inserted in T-vector (Figure 2). The supposed amino acid sequence was highly homologious with that of wheat and maize registered in GenBank after sequencing (Figure 3). So, this fragment was thought to be from CaM clealy, and could be used for gene expression analysis.
|
|
|
|
|
|
1.2 Effects of Ca2+ regulator on CaM gene expression in G. ‘Amaranth’ treated with ethylene
1.2.1 Analysis of expression characteristics of CaM by RT-PCR
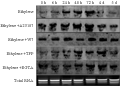
The RT-PCR results showed that the expression abundance of CaM increased in the beginning, and then became lower during the first 7 days flowering-inducing treated with ethylene or ethylene and Ca2+ regulator. However, only treated with ethylene, the expression of CaM increased at 24 h sharply; Treated with ethylene and A23178 (Ca2+ accelerator), the peak expression increased obviously early at 6 h; When treated with ethylene with W7 or TFP (Ca2+ agonist), or ethylene with EGTA (Ca2+ chelant), the expression of CaM increasing remarkable delayed to 72 h or later (Figure 4).
|
|
1.2.2 Northern blot analysis of the expression characteristics of CaM
The results of Northern blot analysis also indicated that expression abundance of CaM increased in the beginning, and then became lower during the first 5 days flowering-inducing treated by ethylene or ethylene and Ca2+ regulator. Only treated by ethylene,he expression of CaM increased sharply at 24 h. Treated by ethylene and A23178 (Ca2+ accelerator), the peak expression increased obviously early at 6 h. When treated by ethylene with W7 or TFP (Ca2+ agonist), the expression of CaM increasing remarkable delayed to 48 h or later. When treated by ethylene with EGTA (Ca2+ chelant) the expression of CaM increasing evidently delayed to 72 h (Figure 5).
|
|
1.3 Effect of Ca2+ regulator on G. ‘Amaranth’ flowering
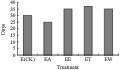
We investigated the flower process by using anatomy methods. The results indicated that ethylene with A23178 could advance the flowering formation 5 d early than that only treated by ethylene. However, Ca2+ agonist and Ca2+ chelant both could delay the flowering formation (Figure 6).
|
|
2 Discussion
Calcium (Ca2+) is not only an important nutrient element, but also plays important roles in plant developmental via a group of Ca2+ target proteins, including CaM and Ca2+-dependent protein kinases (CDPKs).
Flowering is the key physiological feature of plants. Early reports indicated that the CaM which has the most content at the initiation of flower mainly involved in the launch of flower buds in Pyrus pyrifolia Nakai cv. Huanghua (Peng et al., 1998). Ca2+ regulators, including Ca2+ accelerator, Ca2+ agonist and Ca2+ chelant, all affected the flower bud differentiation of strawberry (Luo et al., 2001). In this study, we isolated CaM gene fragment from G. ‘Amaranth’ which had high homology with those CaM registered in GenBank. During the G. ‘Amaranth’ flower process induced with ethylene, the expression changed like unimodal curve, the maximum value appeared at 24 h. A23178, Ca2+ accelerator, brought the maximum value forward to 6 h, while the Ca2+ agonist and Ca2+ chelant both delayed it to 72 h or later. Accordingly, the Ca2+ regulators affected the formation time of floral organ. Ca2+ accelerator promoted the formation of floral organ, but others delayed the floral organ development. Therefore, Ca2+ regulators might affected the Ca2+ contents (Peng et al., 1998; Yi et al., 2011), which influence the expression of CaM and CaM contents accordingly (Yi et al., 2011), and finally affected the flower process of G. ‘Amaranth’ (Yi et al., 2010). On the other hand, Ca2+ accelerator alone could not induce flower of G. ‘Amaranth’ (data not shown), and Ca2+ agonist and Ca2+ chelant could not inhibit the flower induced with ethylene completely, just delay flower. Consequently, Ca2+ regulators had an important regulated role in the flower induction with ethylene of G. ‘Amaranth’, but not decisive effect. It meant that ethylene could not induce the G. ‘Amaranth’ flower through Ca2+-CaM system. So, the regulating pathway and molecular mechanism of flower induction of bromeliads with ethylene need intensive study.
3 Materials and Methods
3.1 Materials
G. ‘Amaranth’ with 20 leaves, 11 month after transplanting from culture bottle, and cultured in the greenhouse of Tropical Crops Genetic Resources Institute, Chinese Academy of Tropical Agricultural Sciences.
Reagents ethylene (40%) was made in China, and Ca2+ accelerator A23178, Ca2+ agonist W7, TFP and Ca2+ chelant EGTA were bought from Sigma Company.
|
|
Treatment methods: This experiment was arranged 5 treatments with 10 plants respectively (Table 1). Firstly, we got rid of the water in the rosulated leaf 1 d before treatment. Filling 5 mL ethylene solution into the center of rosulated leaf at 9:00 pm and discarded the solution after 24 h, and washed with water. The leaf and shoot tip tissue were taken at 0 h, 1 h, 6 h, 24 h, 48 h, 72 h, 4 d and 5 d respectively and soon freeze in nitrogen, and then stored at -80℃.
3.2 Cloning of CaM gene
Total RNA from young leaf and shoot tip was extracted by using CTAB method, and cDNA was synthesized using SuperScript™â…¢ Reverse Transcriptase (Invitrogen). The cDNAs were used for PCR with gene-specific primer (cam1, 5’-CCAgAgCACCTACCC AgACT-3’ and cam2, 5’-ACCTCggAgCAgATgAcg-3’) designed according to the cDNA sequences of CaM genes in GenBank by Primer Premier 5.0 software. The process of amplification followed as: pre-denaturated 5 min at 94℃, and then consisted of 35 cycles of, 30 s at 94℃, 50 s at 60℃, 90 s at 72℃, and a 10 min extension at 72℃. The PCR products were stored at 4℃.
3.3 RT-PCR analysis of CaM gene
Using β-actin as reference gene, the primers were 5’-CAGTGGTCGTACAACTGGTAT-3’ and 5’-ATCC TCCAATCCAGACACTGT-3’. Primers for CaM were cam1 and cam2, which were gave in previous paragraph. The RT-PCR reaction, with a total volume of 25 μL, consisted of 100 ng cDNA, 1×reaction buffer, 2.5 mmol/L MgCl2, 0.25 mmol/L dNTP, 1 U of Taq DNA polymerase (Fermentas) and 0.5 μmol/L of each primer. The amplification process consisted of 35 cycles of 30 s at 94℃, 50 s at 60℃, 90 s at 72℃, and a 10 min extension at 72℃.
3.4 CaM expression analysis by Northern blot
Total RNA was extracted by CTAB method from young leaf and shoot tip of plants treated with ethylene for 0 h, 1 h, 6 h, 24 h, 48 h, 72 h, 4 d and 5 d, respectively. The RNA was stored at -80℃.
For the Northern blot analysis, 10 μg of RNA were separated in formal dehyde-agarose gels and transferred into Nytran membranes (Schleicher & Schuell) and fasten with Spectroline UV Crosslinker, Select TM Series. We used the cDNA reversed from RNA as template and PCR products as the probe. The PCR products was purified and labeled with DIG High Prime DNA Labeling and Detection Starter Kit â… (Roche) and hybridization, and washing conditions were performed as follows: prehybridized with 10 mL of DIF Easy Hyb solution at 42℃ for 30 min. The labeled probe (25 ng/mL) was denatured at 95~100℃ for 5 min and put on ice immediately. And then translated the probe into the heated hybridization solution and mixed. Discarding the prehybridization solution and adding the hybridization solution with probe and hybridized at 45℃ overnight. The membranes were washed once at 15~25℃ in 2×SSC with 0.1% SDS for 10 min and twice at 65~68℃ for 30 min in 0.5×SSC buffer with 0.1% (w/v) SDS. At last, the hybridization signal was detected with scanner (Typhoon Trio).
3.5 Recording the flowering time
The time that the heart leaves began to change color after ethylene treatment was recorded for 5 plants for each repeat. The average value was used for comparing.
Authors’ contributions
ZYL and LX were responsible for experimental design and the experiment direction; ZLY carried out most of the work; HQC took part in the work; ZYL and LX were responsible for data analysis, paper writing and modification. All of them had read the final version of this paper and agreed with the authors’ credits.
Acknowledgements
This work was supported in part by Natural Science Foundation of Hainan Province Grant 808191 and The Basic Scientific Research Funds Operating Expenses for Central Level Institutes Grant PZS004.
References
Burg S.P., and Burg E.A., 1966, Auxin-induced ethylene formation: Its relation to flowering in the pineapple, Science, 152(726): 1269
http://dx.doi.org/10.1126/science.152.3726.1269 PMid:5937118
Dukovski D., Bernatzky R., and Han S., 2006, Flowering induction of Guzmania by ethylene, Scientia Horticulturae, 110(1): 104-108
http://dx.doi.org/10.1016/j.scienta.2006.05.004
da Gunha G.A.P., 2005, Applied aspects of pineapple flowering, Bragantia, Campinas, 64(4): 499-516
Havelange A., 1989, Levels and ultrastructural localization of calcium in Sinapis alba during the floral transition, Plant and Cell Physiology, 30(3): 351-358
Kuan C.S., Yu C.W., Lin M.L., Hsu H.T., Bartholomew D.P., and Lin C.H., 2005, Foliar application of aviglycine reduces natural flowering in pineapple, HortScience, 40(1): 123-126
Poovaiah B.W., and Reddy A.S., 1993, Calcium and signal transduction in plants, Critical Reviews in Plant Sciences, 12(3): 185-211
http://dx.doi.org/10.1080/07352689309701901 PMid:11540065
http://dx.doi.org/10.1080/713608046 PMid:11540065
Turnbull C.G., Sinclair E.R., Anderson K.L., Nissen R.J., Shorter A.J., and Lanham T.E., 1999, Routes of ethephon uptake in pineapple (Ananas comosus) and reasons for failure of flower induction, J. Plant Growth Regul., 18(4): 145-152
http://dx.doi.org/10.1007/PL00007062 PMid:10688702
Wang R.H., Hsu Y.M., Bartholomew D.P., Maruthasalam S., and Lin C.H., 2007, Delaying natural flowering in pineapple through foliar application of aviglycine, an inhibitor of ethylene biosynthesis, HortScience, 42(5): 1188-1191
Gong M., Li Y., and Cao Z.X., 1990, Calcium messenger system in plant, Zhiwuxue Tongbao (Chinese Bulletin of Batany), 7(3): 19-29
Luo C., Peng S.A., and Ma X.T., 2001, Ca2+—CaM signal system and the flower buds differentiation of strawberry, Southwest Horticulture, 29(1): 2-5
Mao G.H., Song L.X., and Sun D.Y., 2004, Progress of study on calmodulin-binding proteins in plants, Zhiwu Shengli Yu Fenzi Shengwu Xuebao (Acta Photophysiologica Sinica), 30(5): 481-488
Peng S.A., Luo C., and Zhang W.C., 1998, Studies on the change of CAM and nuclear acid during flower differentiation of pear, Huazhong Nongye Daxue Xuebao (Journal of Huazhong Agricultural), 17(6): 570-573
Yi Z.L., Li Z.Y., He T.G., Cong H.Q., Huang M.J., and Xu L., 2011, Effect of four calcium regulators on calcium and calmodulin contents during flower bud differentiation of Guzmania ‘Amaranth’ induced by ethylene, Redai Zhiwu Xuebao (Chinese Journal of Tropical Crops), 32(4): 698-701
Yi Z.L., Li Z.Y., Xu L., and Huang M.J., 2010, Effects of 4 calcium regulators on the floral buds differentiation and contents of endogenous hormones of Guzmania ‘Amaranth’, Xibei Zhiwu Xuebao (Acta Botanica Boreali-Occidentalia Sinica), 30(9): 1837-1843
. PDF(425KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Zhiying Li
. Zilin Yi
. Hanqing Cong
. Li Xu
Related articles
. Guzmania ‘Amaranth’
. Ethylene
. Ca 2+ regulator
. CaM
. Gene expression
Tools
. Email to a friend
. Post a comment