2. Graduate School of CAAS, Beijing, 100081, P.R. China
3. Shandong Corporation of CNTC, Jinan, 250101, P.R. China
4. Yunnan Academy of Tobacco Agricultural Sciences, Yuxi, 653100, P.R. China
 Author
Author  Correspondence author
Correspondence author
Plant Gene and Trait, 2012, Vol. 3, No. 6 doi: 10.5376/pgt.2012.02.0006
Received: 02 Mar., 2012 Accepted: 06 Jun., 2012 Published: 08 Jun., 2012
This genetic map drawn by Mapmaker 3.0 containing 17 linkage groups with 75 SSR markers by using a F2 mapping population derived from the intervarietal cross of Yunyan85 × Dabaijin599 and 91 polymorphic markers identified by SSR molecular marker technique. Four QTLs related to the character of easy curing potential were detected based on this genetic map and the phenotype data by the method of composite interval mapping, which mapped on the linkage group 1, 8 and 9, respectively, with the explainable phenotypic variation of 9.26%, 8.87%, 7.57% and 7.83%. The additive effect values of the four QTLs are all positive, indicating these additive effects should come from one of the parent, Yunyan85.
Tobacco is one of an important cash crop and well known as an important model plant in biotechnology; Tobacco’s genome contains 24 pairs of chromosomes. In the past, researchers have revealed lower genetic diversity in tobacco by using RAPD and AFLP markers, of which has affected the progress of the tobacco genetic map construction.
At present, the linkage maps have been reported in tobacco as follows, Lin et al (2001) built the first tobacco molecular marker genetic linkage map based on the 99 individuals of the F2 plants derived from the cross of tobacco wild species, Nicotiana plumbaginifolia × N. longiflora; Nishi et al (2003) constructed a genetic linkage map of the burley tobacco containing 10 linkage groups by using AFLP technology; Xiao et al (2006) constructed a genetic linkage map of flue-cured tobacco including 27 linkage groups and 169 markers consisting of molecular markers based on ISSR and RAPD molecular markers; Julio (2006) constructed a molecular genetic linkage map of flue-cured tobacco consisting of 18 linkage groups and 138 molecular markers based on 114 flue-cured tobacco recombinant inbred lines by using ISSR, AFLP and SSAP markers; Ma et al (2008) constructed a genetic map containing 26 linkage groups and 112 markers by using flue-cured tobacco and burley tobacco based on SRAP markers and ISSR markers; Bindler et al (2011) constructed a genetic linkage map of 2 318 SSR markers containing 24 linkage groups and using F2 plants derived from a cross of Hicks Broadleaf × Red Russian. Diverse genetic maps can be effectively applied to gene mapping of the quantitative traits of different tobacco cultivars, positional cloning, the studies of comparative genomics and molecular marker-assisted breeding, it would be significant to further build the genetic linkage map of tobacco in different cultivating types.
Easy curing potential is one of the important traits of the tobacco, which mainly exists is reflected in yellowing stage of the curing process, representing the degrees of difficulty and synchronization of yellowing and dehydration of tobacco leaves in the curing process; the yellowing characteristics of the tobacco (yellowing index) usually used as a measure of the indicators for the easy curing potential. In general, easy curing potential is defined as the easy curing tobacco leaves being easy to yellow and dehydration, and synchronization of yellowing and dehydration; whereas it is defined as not easy to cure.
At present, there is little researches in tobacco QTL mapping. Xiao et al (2008) used DH population to conduct the QTLs for the traits of the total sugar, nicotine, and potassium in flue-cured tobacco, detected seven QTLs; Chen et al (2009) using AFLP and SRAP molecular markers analyzed the black shank incidence and disease index of burley tobacco for QTL analysis, seven QTLs related to disease resistance to black shank were detected in four linkage groups; Cai et al (2009) also used AFLP and SRAP molecular marker technology to analyze the chemical composition traits and agronomic traits of burley tobacco and detect the 2 QTLs related to the nicotine and total nitrogen and one QTL for total sugar, while one QTL for each trait of plant height, stem girth, internode length, and the length of the middle leaves; Li et al (2011) carried out the six important traits QTL mapping for tobacco nicotine, total chlorine, total potassium, leaf length, angle between stem and leaf, and powdery mildew, which detected two QTLs for nicotine, two QTLs for total chlorine, one for total potassium, four QTLs for leaf length, one QTL for angle of stem and leaf and one for powdery mildew. It has not yet to be reported regarding easy curing potential.
The main purpose of this study is to map the QTL related to the trait of easy curing potential by using F2 population derived from the cross of Yunyan85×Dabaijin599 based on SSR molecular marker genetic linkage map of the flue-cured tobacco, in order to speed up the breeding process for breeding flue-cured tobacco cultivars with excellent easy curing potential trait.
1 Result and Analysis
1.1 The construction of genetic map
Ninety-one pairs of polymorphic SSR markers were screened from 1 900 pairs of the tested SSR primers, which accounts for 4.79% polymorphism rate. 20 of 91 pairs of SSR markers exhibited segregation distortion that was nor consistent with the Mendelian laws. The genetic linkage map of the flue-cured tobacco was constructed that consisted of 17 linkage groups with 75 SSR markers, there were 16 pairs of molecular markers didn’t join to the map. The total genetic distance of the map is 672.2 cM, and the genetic distances of linkage groups from the shortest to the longest are 0.6 cM to 186.5 cM; the average distance between the marks is 11.60 cM, the shortest is 0.6 cM, the longest is 43.9 cM. The number of markers on linkage groups from at least two to the maximum 16; distorted markers are mainly scattered in the linkage groups No.1, No.9 and No.16, while there is no distorted markers in the linkage group No.7.
1.2 The co-relationship of the trait of easy curing potential (yellowing index) and genetic analysis
By using T-test, the final results showed that there was highly significant differences of yellowing index between the P1 and the P2 (P<0.01). F2 populations presented obviously differences in yellowing index ranged from 10.48 to 30.48, 17.93 in average and 4.190 for the standard deviation, showing genetic characteristics of quantitative trait with continuous distribution. The distribution of F2 population with -0.099 39 for kurtosis and 0.654 8 for skewness, of which has less than 1.0 of the absolute value, is a typical distribution of quantitative traits suitable for QTL Mapping. Data are shown in Table 1 and Figure 1.
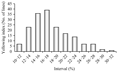 Figure 1 Frequency distribution of yellowing index in F2 population |
Table 1 Yellowing index presented in parents and distributed in F2 population |
1.3 QTL analysis of the easy curing potential
QTL analysis of the easy curing potential in the flue-cured tobacco was conducted using composite interval mapping methods based on the genetic map; four QTLs related to the traits of the yellowing index were detected, which were located in the linkage group of the c1, c8 and c9 (Table 2; Figure 2), thereinto, qECP-1-1 is mapped on the c1 linkage group and located on the markers between Tp179 and Thdp023, and the genetic distance up to the Thdp023 is 10.99 cM; the qECP-8-1 is mapped on the c8 linkage groups and located on the markers between Sca87 and Tp164b, and the genetic distance up to the Sca87 is 2.01 cM. The qECP-8-2 located in c8 linkage groups the located between to marker Tp069 and Sca602, with the distance between the Tp069 0.01 cM. The qECP-9-1 is mapped on the c9 linkage groups and located on the markers between Sca901 and Tep1089, and the genetic distance up to the Sca901 is 0.1 cM. Four QTLs have positive additive effects that indicated the synergistic additive effect for improving easy curing potential comes from Yunyan85, the additive effect of qECP-8-2 should be the maximum value of 0.963 4; whereas the dominance effects of the four QTLs were negative, which implied that the four QTL were negative roles to the dominant gene expression. The explained phenotypic variation of the four QTLs were 9.26%, 8.87%, 7.57% and 7.83%, respectively, of which qECP-1-1 is the largest and qECP-8-2 is the smallest.
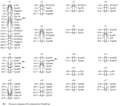 Figure 2 Molecular linkage map of flue-cured tobacco and QTL mapping for the trait of easy curing potential |
Table 2 QTLs and their effects for easy curing potential in flue-cured tobacco |
2 Discussions
Constructing molecular linkage map is the basis for mapping quantitative trait and molecular marker-assisted selection breeding. Tobacco is not plentiful of the polymorphism of molecular markers resulting from the narrow genetic background. Therefore, there is few number of molecular genetic map having been constructed, and most of the genetic maps were constructed by using the markers with poor stability and low polymorphism, such as RAPD, RFLP and other markers. In this study, SSR marker was employed that have many advantages of co-dominant inheritance, plentiful polymorphisms, easy to handle, good repeatability, abundance and even distribution in the genome. Although the constructed map of the flue-cured tobacco only contains 17 linkage groups and limited markers, this map can be further used as frame map for saturating this map by development of new SSR markers.
There is little study on QTL mapping for the important traits in flue-cured tobacco. Easy curing potential is one of the important traits in tobacco; it is the only way to breed the good varieties with fine traits of easy curing potential can produce high-quality tobacco with plentiful flavor and yellow color to meet the needs of the cigarette producers and consumers. QTL mapping for the easy curing potential in flue-cured tobacco would facilitate the genetic improvement of these traits, but it hasn’t yet been reported on gene mapping related to easy cuing potential. In the present study, we adopted the yellowing index as the indicator of easy curing potential in flue-cured tobacco to map the gene linked to the trait and detect four QTLs related to easy cuing potential by using composite interval mapping analysis, sharing the contribution rate of 9.26%, 8.87%, 7.57% and 7.83%, respectively. This would be the important clues to in-depth understanding the genetic basis of the flue-cured tobacco, which will be helpful to fine QTL mapping of easy curing potential.
In general, the gene effect controlling quantitative trait is considered to be similar, and same directional, as well as the number of genes are considered to be multiple, this assumption would be necessary for traditional genetic analysis but it is an idealized model. In fact, the expression of the quantitative trait is the result of interaction of many QTL with the different effects and different directions (Zhang et al., 2004). In this study four QTLs with positive additive effects would be conducive to pyramid the favorable genes to obtain new hybrid combination with atavistic and transgressive inheritance.
At present, the vast majority of studies on quantitative trait still remain on some basic aspects such as the marker identification, positioning, and mapping. The expectation is still not yet to be realized to improve breeding efficiency and develop lines or varieties at large-scale by using marker-assisted selection. The reason might be lack of the integration between marker identification and assisted breeding in previous studies on these two important aspects (Fang et al., 2002). In this study, there were two QTLs tightly linked with molecular markers, the QTL qECP-8-2 located on linkage group 8, which closely linked to Tp069 with genetic distance 0.01 cM; the QTL qECP-9-1 located on linkage group 9, which closely linked to the Sca901 with genetic distance 0.1 cM. Two QTLs related to easy curing potential should be further validated to be used for marker-assisted selection breeding, while to further mine and utilize the marker with big contribution rate would be benefit to improvement of the target traits in marker-assisted breeding.
3 Materials and Methods
3.1 Materials
The experiamental materials are the flue-cured tobacco cultivar, Yunyan85, with good easy curing potential and Dabaijing599, with bad easy curing potential, which came from the National Tobacco Interim Germplasm Bank. The F2 mapping population consisting of 176 individuals was derived from the cross of above mentioned parents. SSR primers used in this study employed from the self-developed primers by Tobacco Research Institute of Chinese Academy of Agricultural Sciences and the Tobacco Institute of Agricultural Sciences and the primers published by Bindler (2007).
3.2 Field experimental design
The yellowing index was determined in the seedling stage to represent the indicator of easy curing potential in flue curing tobacco. Experiments in 2011 were carried ut at the Jimo farm of Tobacco Research Institute of Chinese Academy of Agricultural Sciences; P1 (Yunyan85), P2 (Dabaijing599), F1, F2, were sown at greenhouse in the Jimo farm. The tobacco seedlings were transplanted into small pots with the 13~14 cm diameter when the seedlings grew 4 to 5 true leaves; the top spout was trimmed when the 8th leaf has appeared. Three leaves from top to bottom were picked up after 14 day later, and wrapped book-like shape by using newspaper, and placed in a chamber with constant temperature and humidity (temperature 36℃, relative humidity 90%). The proportion of yellowing was measured seven times once every 24 h, and calculated the yellowing index (Takashi and Tajima, 1984, Chinese tobacco, (3): 45-48).
3.3 DNA extraction and SSR analysis
DNA was extracted followed the modified CTAB method (Yang et al., 2005), and its quality detected by agarose gel electrophoresis and stored at -20℃ refrigerator. The PCR reaction system: PCR reaction system of total 15 μL including 1 × PCR buffer solution, MgCl2 1 mmol/L, dNTPs 1.2 mmol/L for each, primer 0.4 μmol/L, 1 U Taq enzyme, 20 ng genomic DNA. The PCR program: pre-denaturation for 5 min at 94℃, and then 35 cycles for denaturation 45 s at 94℃, annealing for 45 s at the temperature 55℃, or 58℃ or 60℃ based on primer annealing temperature), extension for 45 s , finally, extension for 10 min at 72℃.
3.4 Genetic linkage map construction
The linkage map was conducted by using the software Mapmaker 3.0. The steps were as follows: First, all markers grouped (LOD = 3.0) by the "group" command. Each linkage group was sorted by using the "compare" and "order" command; the remaining markers were added to the sorted sequence by using "try" command, and then using the "Kosambi" function transformed the recombinant value into map units (cM), finally a genetic linkage map was built by "map" command.
3.5 QTL Analysis of and naming
QTL analysis software Windows QTL Cartographer2.5 was employed to map QTL related to easy curing potential by using composite interval mapping (CIM) and to detect QTL effect. The parameters for CIM analysis were set as the follows, 2.0 cM of step, the 1 000 times of regression calculation, significant level at 0.01, and 2.0 of the LOD value.
QTL was named following as QTL + trait + chromosome + the number of QTL, of which the QTL with a lowercase "q", the trait shown as abbreviation, if there is one more QTL on the same chromosome, a number of different sites of the QTL on same chromosome would be assigned to the number "1", "2 "," 3 "in order to distinguish the different locus (McCouch et al., 1997).
Authors' Contributions
XLT designed and carried out the experiment; ZFZ and XHX conceived this program, directed the experiment, analyzed the data, as well as wrote and modified the manuscript; XWZ helped to analyze the data; NCW and JLX was responsible for the material; WFW and BGX helped design the primers; CYW, JR and XWH took part in the experiment. All authors have read and approved the final manuscript.
Acknowledgements
This work was co-supported by Science and Technology Key Project of China National Tobacco Corporation, Science and Technology Key Project of Genetic Analysis of Flue-Cured Tobacco Baking Characteristics and QTL Mapping Study of Shandong Province of China National Tobacco Corporation (110 201 002 017 Lu Yan Ke [2011] No. 13).
References
Bindler G., Plieske J., Bakaher N., Gunduz I., Ivanov N., Van der Hoeven R., Ganal M., and Donini P., 2011, A high density genetic map of tobacco (Nicotiana tabacum L.) obtained from large scale microsatellite marker development, Theor. Appl. Genet., 123(2): 219-230
Bindler G., van der Hoeven R., Gunduz I., Plieske J., Ganal M., Rossi L., Gadani F., and Donini P., 2007, A microsatellite marker based linkage map of tobacco, Theor. Appl. Genet., 114(2): 341-349
http://dx.doi.org/10.1007/s00122-006-0437-5 PMid:17115128
Chai C.C., Chai L.G., Wang Y., Xu F.S., Zhang J.J., and Lin G.P., 2009, Construction of genetic linkage map of burley tobacco (Nicotiana tabacum L.) and genetic dissection of partial traits, Zuowu Xuebao (Acta Agronomica Sinica), 35(9): 1646-1654
http://dx.doi.org/10.3724/SP.J.1006.2009.01646
Chen D.W., Chai L.G., Cai C.C., Lin G.P., Wang Y., and Xu F.S., 2009, Construction of genetic linkage map of burley tobacco (Nicotiana tabacum L.) and the QTL analysis of black shank disease, Ziran Kexue Jinzhan (Progress in Natural Science), 19(8): 852-858
Fang X.J., Wu W.R., and Tang J.L., eds., 2002, Molecular marker assistant breeding in crop, Science Press, Beijing, China, pp.121-128
Julio E., Denoyes-Rothan B., Verrier J.L., and Dorlhac de Borne F., 2006, Detection of QTLs linked to leaf and smoke properties in Nicotiana tabacum based on a study of 114 recombinant inbred lines, Molecular Breeding, 18(1): 69-91
http://dx.doi.org/10.1007/s11032-006-9019-0
Li H.L., Chen M.X., Zhou D.X., Chen S.H., Tao A.F., Li Y.K., Ma H.B., Qi J.M., and Guo Y.C., 2011, QTL analysis of six important traits in tobacco, Zuowu Xuebao (Acta Agronomica Sinica), 37(9): 1577-1584
http://dx.doi.org/10.3724/SP.J.1006.2011.01577
Lin T.Y., Kao Y.Y., Lin S., Lin R.F., Chen C.M., Huang C.H., Wang C.K., Lin Y.Z., and Chen C.C., 2001, A genetic linkage map of Nicotiana plumbaginifolia/Nicotiana longiflora based on RFLP and RAPD markers, Theor. Appl. Genet., 103(6-7): 905-911
http://dx.doi.org/10.1007/s001220100618
Ma H.B., Qi J.M., Li Y.K., Liang J.X., Wang T., Lan T., Chen S.H., Tao A.F., Lin L.H., and Wu J.M., 2008, Construction of a molecular genetic linkage map of tobacco based on SRAP and ISSR markers, Zuowu Xuebao (Acta Agronomica Sinica), 34(11): 1958-1963
http://dx.doi.org/10.3724/SP.J.1006.2008.01958
McCouch S.R., Cho Y.G., Yano M., Paul E., Blinstrub M., Marishims H., and Kinoshita T., 1997, Report on QTL nomenclature, Rice Genetic Newsletter, 14: 11-13
Nishi T., Tajima T., Noguchi S., Ajisaka H., and Negishi H., 2003, Identification of DNA markers of tobacco linked to bacterial wilt resistance, Theor. Appl. Genet., 106(4): 765-770
PMid:12596008
Xiao B.G., Lu X.P., Jiao F.C., Li Y.P., Sun Y.H., and Guo Z.K., 2008, Preliminary QTL analysis of several chemical components in flue-cured tobacco (Nicotiana tabacum L.), Zuowu Xuebao (Acta Agronomica Sinica), 34(10): 1762-1769
http://dx.doi.org/10.3724/SP.J.1006.2008.01762
Xiao B.G., Xu Z.L., Chen X.J., Shen A.R., Li Y.P., and Zhu J., 2006, Genetic linkage map constructed by using a DH population for the flue-cured tobacco, Zhongguo Yancao Xuebao (Acta Tabacaria Sinica), 12(4): 35-40
Yang B.C., Xiao B.G., Chen X.J., and Shi C.H., 2005, Genetic diversity of flue-cured tobacco varieties based on ISSR markers, Yichuan (Hereditas), 27(5): 753-758
Zhang H.Y., Chen Q.J., Wang Y.J., Xu Y., and Zhang F., 2004, Identification of QTLs for cucumber poor light tolerance, Fenzi Zhiwu Yuzhong (Molecular Plant Breeding), 2(6): 795-799
. PDF(757KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Xiaolei Tan
. Xiuhong Xu
. Nuanchun Wang
. Xingwei Zhang
. Jie Ren
. Bingguang Xiao
. Jialai Xu
. Weifeng Wang
. Chuanyi Wang
. Xianwei Hao
. Zhongfeng Zhang
Related articles
. Flue-cured tobacco
. Easy curing potential
. QTL
Tools
. Email to a friend
. Post a comment


