Response of Plant Growth Regulators and Nitrogen on Photosynthetic Pigments, Soluble Protein and Yield of Black Gram (Vigna mungo L.) 
2. Professor of Crop Physiology, Department of ACRC, TNAU, Coimbatore–3, India;
 Author
Author  Correspondence author
Correspondence author
Plant Gene and Trait, 2013, Vol. 4, No. 5 doi: 10.5376/pgt.2013.04.0005
Received: 01 Apr., 2013 Accepted: 11 Apr., 2013 Published: 27 Jan., 2004
Investigations were undertaken to study the effect of nitrogen in combination with foliar spray of bioregulators and micronutrients on growth and productivity of CO5 black gram. Photosynthetic pigments and foliage soluble protein content were estimated at different phenological phases of black gram. Seed yield were assessed at the time of harvest. Significant increase in the chlorophyll content of the leaf due to basal application of nitrogen 25 kg per hectare with foliar spray of urea 2% and 0.1 ppm brassinolide. The biochemical constituents such as soluble protein content were also greatly enhanced by the basal application of nitrogen 25 kg per hectare with foliar spray of urea 2% and 0.1 ppm brassinolide treatment.
1 Introduction
Pulses are the most important crops in India because of its low cost and high quality protein. They play a major role in providing a balanced protein component in the diet of the people. Pulses contain a higher level of quality protein, nearly three times as much as cereals; therefore they are the cheapest and rich source of protein and essential amino acids and thus share a major protein of the vegetarian diet. Besides, the crops enrich the soil fertility and health in terms of addition of nitrogen and organic matter. Among pulses, black gram (Vigna mungo L. Hepper), occupies a unique place for its use as vegetable, and it is grown both as pure and mixed crop along with maize, cotton, sorghum and other millets. It is also known as urd bean, and it is an important pulse crop grown all over the world. It is a major component of the daily Indian diet and serves as a rich protein source (23.9%) besides, it also contains 60.4 per cent carbohydrates. As per the World Health Organization every man needs 80 g of pulses per day and as per the Indian Council of Medical Research, every man needs minimum consumption of 47 g of protein per day to meet requirement of the body. But at present, the per capita availability of pulses is only 30~35 g per day. Therefore, there is a need for three fold increase in pulse production as that of current production. Black gram is indeterminate in its flowering and fruiting habits and there is a competition for available assimilates between vegetative and reproductive sinks. There is limitation of source (leaves) particularly at flowering and fruiting stage. Hence, there is a need to improve LAI and LAD. Being a C3 plant, CGR and RGR are relatively less than cereals and the major yield components are pods per plant, seeds per plant and test weight of seeds. Apart from this genetic makeup, the major physiological constraints limiting its production are flower drop and fruit drop (Ojeaga and Ojehomon, 1972). This performance of the crops can be overcome by foliar application of growth regulating chemicals at the crucial stages of the crop, which is one of the latest trends in agriculture. The growth regulating chemicals bioregulators can improve the physiological efficiency including the photosynthetic ability of crop and play a significant role in improving the productive potential of the crop. With this above background, the present investigation was carried out.
2 Materials and methods
The present investigation was undertaken under field condition to study the effect of nutrients and plant growth regulators on growth and productivity of black gram variety CO5. The research experiment was carried out at Millet Breeding Station, Tamil Nadu Agricultural University, Coimbatore during July to October, 2007. Growth regulators like Naphthalene Acetic Acid (NAA), Salicylic Acid (SA), Cycocel (CCC), Brassinosteriod (BR), Humic Acid and the nutrients such as Nitrogen, DAP, Boric Acid, Ferrous Sulphate, Zinc Sulphate were used. The data were statistically analyzed with the Design of Randomized Block Design with three replication and the Plot size of 4 x 3 m with Spacing of 30 x 10 cm. in this research study having nine treatments and the details are, T1 - Control , T2 - N 25 Kg/ha + Urea 2 % + NAA 40 ppm, T3 - N 50 Kg/ha + CCC 200 ppm, T4 - N 25 Kg / ha + Urea 2 % + CCC 200 ppm, T5 - N 25 Kg/ha + Urea 2 % + Humic acid 0.1 %, T6 - N 25 Kg/ha + Urea 2 % + Salicylic acid 100 ppm, T7 - N 25 Kg/ha + Urea 2 % + Brassinosteriod 0.1 ppm, T8 - N 25 Kg/ha + Urea 2 % + ZnSO4 0.5% + FeSO4 0.5 + Borax 0.2%, T9 - N 25 Kg/ha + Water spray.
|
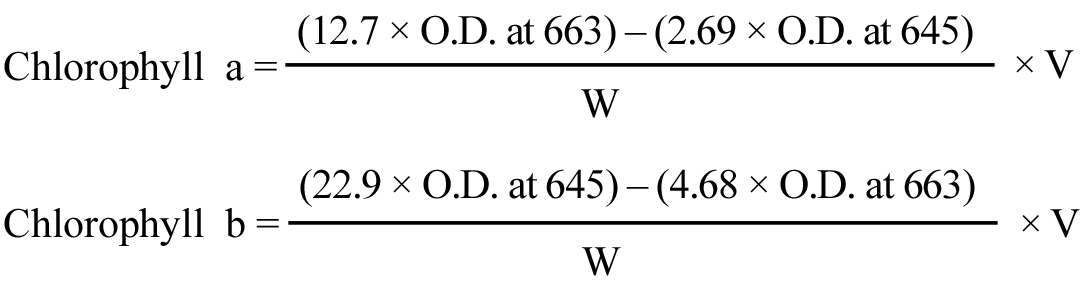 |
Chlorophyll a, b and total chlorophyll were estimated in physiologically active (index) leaf as per the proceture of Yoshida et al., (1971) and expressed as mg/g fresh weight.
The content of soluble protein was estimated from the leaf samples following the method of Lowry et al. (1951) and expressed as mg/g fresh weight.
3 Result
3.1 Photosynthetic pigments (Chlorophyll ‘a’ and ‘b’)
3.1.1 Chlorophyll 'a' content (mg/g)
The data on chlorophyll ‘a’ (mg) content of black gram variety CO5 as influenced by the nutrients and plant growth regulators at various growth stages is presented in Table 1. In general, the chlorophyll 'a' content was found to be maximum at the pod filling stage (60 DAS), with a decline towards the harvest stage. The treatment T7 (N 25 mg/g+ urea 2% + 0.1 ppm BR) recorded the maximum chlorophyll ‘a’ content of 0.96 mg, 1.26 mg, 1.84 mg and 1.45 mg at vegetative, flowering, pod filling and harvest stages, respectively.
|
.png) Table 1 Effect of nitrogen nutrition and growth regulators on Chlorophyll ‘a’ content (mg/g) in black gram at different growth stages |
3.1.2 Chlorophyll 'b' content (mg/g)
Foliar spraying of nutrients along with growth regulators had significant effect on the chlorophyll 'b' content values at all the stages of crop growth (Table 2). The results indicated an increase in chlorophyll 'b' content from vegetative (30 DAS) to pod filling stage (60 DAS), which showed a decline trend at harvest stage. The values ranged from 0.29 to 0.82 mg, 0.40 to 0.94 mg/g, 0.48 to 1.04 mg/g and 0.38 to 0.99 mg/g at vegetative, flowering, pod filling and harvest stages, respectively. The effect of brassinolide was very evident from the high values recorded in this treatment T7 (N 25 mg/g+ urea 2% + 0.1 ppm BR) at all the stages of growth. The control treatment recorded significantly lower values (0.29, 0.40, 0.48 and 0.38 mg/g).
|
.png) Table 2 Effect of nitrogen nutrition and growth regulators on Chlorophyll ‘b’ content (mg/g) in black gram at different growth stages |
3.2 Soluble protein (mg/g)
The data on soluble protein content (mg) of leaves was recorded at various stages of crop growth viz., vegetative (30 DAS), flowering (45 DAS), pod filling (60 DAS) and harvest stage (Table 3). The soluble protein content increased up to pod filling stage (60 DAS) with a decline at harvest stage, which ranged from 12.8 to 15.4 mg/g, 15.2 to 19.5 mg/g, 19.1 to 26.7 mg/g and 10.0 to 16.4 mg/g, respectively. The treatment T7 (N 25 mg/g+ urea 2% + 0.1 ppm BR) registered more soluble protein content of 15.4 mg/g, 19.5 mg/g, 26.7 mg/g and 16.4 mg/g at all the stages of crop growth.
|
.png) Table 3 Effect of nitrogen nutrition and growth regulators on soluble protein content (mg/g) in black gram at different growth stages |
4 Discussion
4.1 Photosynthetic pigments
The increase in chlorophyll content reflects increased PS II photochemistry, photosynthates production and dry matter accumulation. The result of the present study indicated that the fractions of chlorophyll, a and b also total chlorophyll increased up to pod filling stage and declined at the time of harvest. Chlorophyll accumulation in leaves was double time higher between flowering and pod filling stages, as compared to vegetative stage, whereas, for chlorophyll b, the maximum accumulation was noticed at vegetative stage itself. The total chlorophyll content, however, reflected the trend of chlorophyll a content. At the time of harvest, chlorophyll a and total content showed about 30 per cent reduction, whereas chlorophyll b showed only 10 per cent reduction. This decline was related to the activation of chlorophyllase enzyme, as the result, the water soluble porphyrin fragments were exported from chloroplast to vacuole, thus there was a loss in leaf greenness (Matile et al., 1982). The nutrients and plant growth regulators played a productive role in upregulating the enzymes involved in chlorophyll synthesis. The treatmental combination N (25 mg/g) + BR (0.1 ppm) + Urea (2%) caused a remarkable increase in a, b and total content of the leaves at all the stages of the growth, as observed in the present study. This finding was strongly supported by the results of Prabakaran (2002) in black gram. Mitra and Ghildyal (1987) opined that the supply of nitrogen through foliage is essential during pod developmental stage for the maintenance of high rate current photosynthesis. Kulaeva et al., (1991), however, specified the role of BR in inducing chlorophyll synthesis, through the activation of enzyme proteins. In mung bean, the enhancement in chlorophyll content by BR treatment had been attributed to several factors including inhibition of senescence and enhanced uptake of iron (Bhatia and Jatinder kaur, 1997). Similarly in wheat high photosynthetic rate induced by BR application was directly related to enhanced chlorophyll content of the leaf (Sairam, 1994). In addition to the combination with BR 0.1 ppm, CCC 200 ppm along with Urea 2 per cent also revealed its significant effect in enhancing the chlorophyll content of the present study. Ameregouda et al. (1994) supported this finding with the result that in wheat, foliar spray of CCC was found highly effective in increasing the chlorophyll content of the leaf. Similar finding made by Sumathi (2005) in pigeon pea was also in close confirmity with the results of the present study.
4.2 Soluble protein
The soluble protein content of the leaf, being a measure of RuBP carboxylase activity was considered as an index for photosynthetic efficiency. These were reports that RuBP-case enzyme forms nearly 50 per cent of the soluble proteins in leaves of many plants (Joseph et al., 1981). Diethelm and Shibles (1989) opined that the RUBISCO content per unit leaf area was positively correlated with that of souble protein content of the leaf. Vidyavardhini and Seeta Rama Rao reported that foliar application of brassinolide and 24-epibrassinolide at 0.5ppm to 3.0 ppm remarkably increased the soluble protein content of the leaves in groundnut. This finding strongly supported the results of the present study, which indicated that the treatmental combination N (25 kg/ha) + BR (0.1 ppm) + Urea (2%) enhanced the soluble protein content by more than 25 per cent at the time of flowering over control. This effect of BR was explained by Gregory (1981) that increased level of soluble protein content in the brassinolide treated plants might be due to induction of specific metabolic changes and increased protein synthesis in cells. Kalinich et al. (1985) reported a significant increase in RNA and DNA polymerase activity and the synthesis of RNA, DNA and protein in brassinosteroid treated bean and mungbean, which might be the reason for increase in soluble protein content in these crops. The other treatmental combination with CCC 200 ppm, of the present study also showed its effectiveness in enhancing soluble protein content, particularly, at the flowering stage of the crop. In supporting this finding, Krishchenko et al. (1983) reported that soluble protein content in wheat increased remarkably during tillering and flowering stages as the result of CCC application. Following this, El-Tahawari et al. (1983) recorded an increased protein synthesis and amino acid contents of all plant organs of Phaseolus vulgaris due to CCC application. In rye plants also increased leaf protein synthesis was recorded by Kozyreva (1985).
References
Ameregouda A., Chetti M.B., and Manjunath S., 1994, physiological basis of yield variation due to application of different chemicals in wheat, Ann. of Pl.Physiol., 8(1): 24-28
Bhatia D.S. and kaur J., 1997, Effect of homobrassinolide and humicil on chlorophyll content, hill activity and yield components in mungbean (Vigna radiata (L.) Wilczek), Phytomorphol., 47: 421-426
Diethelm R., and Shibles R., 1989, Relationship of enhanced sink demand with photosynthesis and amount and activity of Ribulose-1, 5 biphosphate carboxylase in soyabean leaves, J. Plant Physiol., 134: 70-74
http://dx.doi.org/10.1016/S0176-1617(89)80204-4
EL-Tahawari B.S., Diab M.A., Habib M.A., and Draz S.N., 1983, Protein biosynthesis in plants of phaseolus vulgaris as affected by CCC, Pl. Growth Reg. 10(5): 1635
Gregory L.E., and Mandava N.B., 1981, The activity and interaction of brassinolide and gibberelic acid in mung bean epicotyls, Physiol. Plant, 54: 239-243
http://dx.doi.org/10.1111/j.1399-3054.1982.tb00253.x
Joseph M.C., Ramdall D.D., and Nelson C.J., 1981, Photosynthesis in polyploid tall fescue, II. photosynthesis and Rubp case of polyploid tall fescue, Plant Physiol., 68: 894-898
http://dx.doi.org/10.1104/pp.68.4.894
PMid:16662021 PMCid:426008
Kalinich J.F., Mandava N.B., and Tod Hunter J.A., 1985, Relationship of nucleic acid metabolism to brassinolide induced responses in leaves, J. Plant Physiol., 120: 207-214
http://dx.doi.org/10.1016/S0176-1617(85)80107-3
Kozyreva T.F., 1985, Increasing cold resistance of rye during over wintering, Dushanbe.Tajik SSR., 40-41
Kulaeva O.N., Bu rkhanov E.A., Fedeira A.B., Khochloa V.A., Bokebayeva G.A., Vorbrodt H.M., and Adane G., 1991, Effect of brassinosteriods on protein synthesis and plant cell ultrastructure under stress condition, In: Gutlert H.G., Vok T., and Adanus G., eds., Brassinosteroids, Chemistry, Bioactivity and Application, Amer. Chem. Soc. Washington, D.C, U.S.A., pp.141-155
Lowry A.H., Brough N.T.R., Fait L.A., and Randall R.J., 1951, Protein measurement with folin phenol reagent, J. Biol. Chem., 193: 262-275
Matile P., and Marschner W., 1982, Chloroplast senescence, In: Baker N.R., and Thomas H., eds., Crop photosynthesis, spatial and temporal determinants, Amsterdam, Elsevier, PP.413-440
Mitra R., and Ghildyal, 1987, Nitrogen: The major limiting factors for mungbean yield, Symp., Bangkok, 16-20:245-251
Ojehomon O.O., 1972, Fruit absicission in cowpea (Vigna ungiculata (L) wasp.), J.Exp.Bot., 23: 751-761
http://dx.doi.org/10.1093/jxb/23.3.751
Sairam R.K., 1994, Effect of homobrassinalide application on plant metabolism and grain yield under irrigated and moisture stress conditions of two wheat varieties, Plant Growth Regul., 14: 173-181
http://dx.doi.org/10.1007/BF00025220
Yoshida S.D., Foron A., and Cock J.H., 1971, Laboratory Manual for Physiological Studies of Rice, IRRI, Philippines, pp.36-37
. PDF(202KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. K. Krishna Surendar
. S. Vincent
. Mallika Vanagamudi
. H. Vijayaraghavan
Related articles
. Black gram
. PGR
. Nitrogen and yield
Tools
. Email to a friend
. Post a comment


