Transformation of Cucumber Flowering Integrator (CFL Gene) into Cyclamen persicum Mediated by Agrobacterium 
2. Realty Service Center, Hangzhou Normal University, Hangzhou, 310036, P.R. China
 Author
Author  Correspondence author
Correspondence author
Plant Gene and Trait, 2011, Vol. 2, No. 1 doi: 10.5376/pgt.2011.02.0001
Received: 22 Nov., 2011 Accepted: 08 Dec., 2011 Published: 17 Dec., 2011
Xu et al., 2011, Transformation of Cucumber Flowering Integrator (CFL Gene) into Cyclamen persicum Mediated by Agrobacterium, Molecular Plant Breeding, 9(5): 629-634 (doi: 10.3969/mpb.009.000629)
By employing cyclamen young leaves, petioles and callus from Cyclamen persicum as explants, transformation of cucumber flowering integrator (CFL gene) into the genome of Cyclamen persicum mediated by Agrobacterium tumefaciens was conducted to compare the transformation efficiencies of different explants and to optimize the sonication-assisted transformation procedure mediated by Agrobacterium. The results demonstrated that the CFL gene was integrated into the genome of regenerated Cyclamen plants, which were validated by PCR and RT-PCR and by GUS histochemical assay. The results also showed that young leaves were the best receptor for transformation among employed explants, which the GUS-positive rates of regenerated plants can reach 27.25%. Furthermore, GUS positive rate can be increased four times by using the sonication-assisted transformation procedure. In this study, we successfully introduced the CFL gene into the genome of Cyclamen persicum by using modified high-efficiency genetic transformation system, which might be an alternative way for developing early flowering Cyclamen variety.
Cyclamen (Cyclamen persicum) is one of the world's top ten potted flower plant, native to the Mediterranean region, now widely cultivates throughout the world with highly ornamental features due to its flower shapes, colors, leaves, vein patterns and diverse stripes. It is also very popular indoor potted flowers in winter, particularly in New Year's Day and Spring Festival because of its long flowering spans in less flower-supply winter season. However, due to influences of the environment, cultivation techniques and other factors, the excellent characters of cyclamen varieties is yet to be exhibited, usually vegetative growth stage tends to be extended, which not only will miss the season promotion, but also increases the cost of production for those cyclamen mainly selling in the New Year and Chinese New Year. Currently, the general measures to control cyclamen flowering periods are mainly focus on sowing in advance, germination in high mountain, and hormone treatment methods (Zhao et al., 1995; Hu and Liu, 2008). Although the effects of early sowing and high mountain germination were good for flowering control, the costs increased in production, while the effects of hormone treatment was not yet stable or not too obvious. Therefore, it would be very important to develop new varieties of cyclamen to short vegetable growth stage. By using genetic engineering technology to introduce enhancing flowering gene into the genome of cyclamen might be an effective way to regulate the flowering period, which will reduce the costs of products and in increase competition in market, leading to greater economic benefits. Figure 1 The sensitivity of different explants in Cyclamen persicum to hygromicin
Flowering is a complex process that is the results of comprehensive regulation of flowering-related genes. LEAFY (LFY) gene, belonging to flowering integrator gene, is flower meristem-specific gene, which is in the crucial position of the regulation network of plant flowering as well as plays an important roles in the transform from the vegetative to the reproductive (Wang, 2004). The activity of LFY gene is quite conservative among the species with the distant genetic relationship (Weigel and Coupland, 1995), therefore, through the introduction of LFY homologous gene, it is possible to promote early flowering in plants. Weigel and Coupland (1995) introduced the Arabidopsis LFY gene into European Populus hybrids, having the plants that originally need 8 to 20 years flowering within six months; Shao et al (1999) introduced Arabidopsis gene LFY into chrysanthemum plants leading to flowering ahead of 60~70 d transgenic plants. CFL gene was cloned from cucumber homologous to LFY genes. Northern hybridization showed that it is mainly expressed in flower buds and young leaves, suggesting that genes might play a role in transformation in the vegetative growth and flowering (Liu et al., 1999). In experiment of the CFL genetic transformation in gloxinia, transgenic plants can be flowering ahead 26~32 d earlier, shortening a quarter in vegetable stage comparing to non-transgenic plant (Zhang et al., 2008). At present, Studies on genetic transformation of cyclamen have been some reported, such as genetic transformation by changing the color of cyclamen (Zhao, 2005), increasing the resistance of cyclamen to stresses (Chen et al., 2008; Zhu and Ma, 2009). There is no report to change the flowering of cyclamen by using exogenous genes yet. In this study, we attempted to establish an efficient genetic transformation system to obtain the modified cyclamen with CFL gene which leads to change flowering patterns, providing an alternative to developing new varieties of early flowering cyclamen.
1 Results and Analysis
1.1 Determination of hygromycin selection pressure
Hygromycin (Hygromycin B, Hyg) has a strong inhibitory effect on cyclamen leaves, petioles and callus growth and regeneration. As shown in Figure 1, three kinds of explants cultured in the medium without Hyg in the dark for 30 d, survival rate of leaf and callus reached 100% as well as survival rate of petiole was 80%. In the 5 mg/L Hyg treatment conditions after culture in dark for 30 d, leaves were becoming serious browning and their regeneration abilities were inhibited, only a very few leaf explant happened to be regeneration; mortality rate of browning calli were slightly lower than that of the leaves, while petiole have been brown and dead. When Hyg concentration continues to increase, the leaves and calli were both all brown and dead. So in this experiment, 5 mg/L of Hyg as initial screening concentration of cyclamen leaves and callus, 4 mg/L of the Hyg as the initial concentration of cyclamen petioles.

1.2 Comparisons of transformation efficiency in different explants of cyclamen
Using the leaves, petioles and calli as transformation receptors cultured on selection medium for 60 d or so, the resistant buds on the leaves and petiole directly appeared (Figure 2D; Figure 2E). Much of resistant buds formed fascicle on the leaves, while calli can generate differential resistant buds (Figure 2F). However, in the same selective medium, different receptors of cyclamen had quit different transformation efficiency. As shown in table 1, for the positive rate in terms of GUS to transformation explants, calli were the highest, reaching 81.34%, leaf and petiole were 18.66% and 10.66%, respectively; For the positive rate of GUS to leaf generation, leaves were highest up to 27.25%, calli ranked second, up to 14.63%; petiole placed lowest, only 6.39%. So, for GUS-positive rate of the regenerated plants, leaves were the best in the three transformation explants.
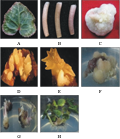 Figure 2 Transformation receptors and formation of resistant plants in Cyclamen persicum |
Table 1 The transformation efficiency of different transformation receptors in Cyclamen persicum |
1.3 Effects of ultrasonic treatment on the transformation efficiency of cyclamen leaves
As results mentioned above, leaves as explants were the best one in tested three receptors, so in this experiment we optimized the transformation system by using leaves as only receptor. After selective cultured in 60 days, the results showed that the appropriate ultrasonic treatment can significantly increase the transformation efficiency based on observation of morphology and GUS staining (Table 2). In both experiments, ultrasonic treatment with 60 seconds was the best, the highest rate of leaf GUS-positive reached 83%, continuing increasing the ultrasonic treating time leaded to a serious leaf damage and decrease of transformation efficiency. Therefore, ultrasonic treatment with 60 seconds for leaves would be more appropriate to increase the transformation efficiency.
Table 2 The effects of ultrasonic treatment on transformation efficiency of leaves in Cyclamen persicum |
1.4 GUS staining analysis of the resistant plants
Cyclamen calli and leaves from 41 resistant plants were used to analyze GUS histochemical staining, the results showed that 15 plant lines appeared blue reaction, while leaves and calli no blue reaction in control plants (Figure 3).
 Figure 3 The GUS histochemical staining of CFL transformed Cyclamen |
1.5 Resistant plants by PCR and RT-PCR detection
The five plants with GUS positive were randomly selected to be validated by using CFL gene-specific primers. PCR amplification results (Figure 4) showed the selected five plants were amplified approximately the target band of 637 bp in length, while the wild-type control did not be amplified any bands, which indicated that the CFL gene should be integrated into the genome of cyclamen.
Figure 4 PCR assay of CFL transformed Cyclamen |
In order to validate whether the introduced foreign gene was expressed in the cyclamen, we extracted the total DNA from the above mentioned PCR positive five plants to perform RT-PCR detection by using wild-type as control. Results (Figure 5) confirmed that five plants were amplified CFL gene target band, which suggested that exogenous gene should express in cyclamen plants.
Figure 5 RT-PCR assay of CFL transformed Cyclamen |
2 Discussions
In existing reports on cyclamen genetic transformation, transformation efficiency of cyclamen was quit low (Aida et al., 1999; Li, 2004; Chen et al., 2008). Our study found that the resistant calli of cyclamen being difficult to differentiation would be a major reason besides cyclamen itself is more difficult to be genetically transformed. Zhu and Ma (2009) found that hygromycin could inhibite the growth of resistant bud growth of cyclamen, resulting in slowness of bud growth and even termination. Dai et al (2010) also found that explant differentiation was very difficult after the treatment of hygromycin in the experiment of narcissus genetic transformation. In this study, we obtained resistant calli with its creamy white color, texture compacting, growing well, as well as higher frequency of resistant callus by using cyclamen callus transformation system, but the resistant calli were still difficult to differentiate, which indicated that the hygromycin for cyclamen bud differentiation had a strong inhibitory effect. Using leaves as receptor can generate resistant calli and adventitious buds after screening by hygromycin, which made the following differentiation relatively easy, furthermore, adventitious buds regenerating from the leaves can directly skip the step of callus to reduce the false positive rate. Therefore, three kinds of receptors, callus, leaves and petiole, the leaf would be the best transformation receptors.
Ultrasonic treatment has some effects of cavitation and mechanical injury on the cells, which may result in forming many tiny holes in the surface and inside of the leaf to create the conditions for the exchange of intracellular material. Under the suitable conditions of the ultrasonic processing power and time, ultrasonic treatment could not affect the activity of cells (Wang and Fang, 2002). Producing these subtle injuries will increase the cell contacting area with the strain of Agrobacterium tumefaciens, which significantly increased the ability of Agrobacterium infection. At present, Sonication-assisted transformation has been applied in the genetic transformation of plants. The transformation efficiency by assisted by ultrasonic treatment was significantly higher than that only using Agrobacterium-mediated transformation (Trick and Finer, 1998; Yao and Wang, 2001; Jiang et al., 2004). Our results showed that ultrasonic treatment can significantly increase the transformation efficiency of cyclamen. The most suitable conditions of treatment would be under the power of 100 W for 60 seconds, which could obtain stable resistant tissues of cyclamen with positive rate of 83%, the transformation efficiency was increased four times than that without ultrasonic treatment.
Excellent resistant screening method has been very important for resistant plants. In view of hygromycin's effects on bud differentiation restraining or bud varying, in this study, we adopted the procedures that removed hygromycin pressure in the medium once the formation of resistant buds, and then detected each line until the buds growing up. In fact, cyclamen differentiation usually generates the clustering seedlings rather than individual one, which would be increased the risk of chimeric formation. To minimize the formation of chimeras, we picked up a single seedling leaf to be detected for maximum acquiring the homozygous lines. We also found that it was more difficult to induce the calli and adventitious buds from leaves of transgenic plants than that of the leaves of wild-type plants, not only with long inducing time and less amount of differential buds, but also poor quality. In addition, the survival rate of transplanted transgenic plants were quite low, this may be due to relatively poor growth conditions of the transgenic plant self, as well as to breaking original plant own metabolic balance because of the constitutive expression of CFL gene.
The roles of LFY gene in flower development have been studied more clearly, many LFY homologous genes have been cloned from bryophytes to angiosperms, which laid the foundations for those plants with long vegetative growth to be early flowering by heterologous transformation, especially for some of the economic plants. However, There are a little reports on LFY gene to promote early flowering in the heterologous expression in plants, as yet, only reported in Arabidopsis, poplar, citrus, chrysanthemum, rice, gloxinia (Weigel and Coupland, 1995; Blazquez et al., 1997 ; Shao, et al., 1999; Pena et al., 2001; He et al., 2000; Zhang et al., 2008). In this study, we conducted a useful exploration in cyclamen flowering breeding to lay the foundations for developing novel cultivars with short vegetable growth stage, which would has practical application value to meet the demands of flower markets.
3 Materials and Methods
3.1 Plant material
Cyclamen (Cyclamen persicum) used in this research provided by Key Laboratory of Plant Science and Technology of Hangzhou Normal University, Hangzhou, Zhejiang Province.
3.2 Strains and plasmids
Agrobacterium tumefaciens strain EHA105, plasmid pCAMBIA13011 containing CFL gene, Hyg resistant gene and the GUS gene, kindly donated by Prof. Zhu from Zhejiang University, Hangzhou, Zhejiang Province.
3.3 Culture medium
Callus induction medium: modified MS+2.0 mg/L BA+1.0 mg/L 2,4-D+sucrose 30 g/L+agar 5 g/L; Bud differentiation medium: modified MS+0.1 mg/L BA+30 g/L sucrose+5 g/L agar; Rooting medium: 1/2MS+1.0 mg/L IBA; Selective medium: adding 4 or 5 mg/L tide mold Su and 500 mg/L cefotaxime in callus induction medium and or bud differentiation medium.
3.4 Detection of leaves, petioles and callus to hygromycin sensitivity
Taking sterile cyclamen young leaves, petioles and callus, scratching the tissues prior to placing on the Hyg callus induction medium with Hyg concentrations of 0, 3 mg/L, 4 mg/L, 5 mg/L, 6 mg/L, 8 mg/L and 10 mg/L. After incubating in the dark 30 days, the growth status of the leaves, petioles and calli were observed to calculate survival rate. The lowest Hyg concentration that restrain the receptor's growth was defined as selective pressure. Each treatment was inoculated 30 materials with three repeats.
3.5 Genetic transformation of cyclamen different receptors
Under the sterile conditions, the sterile young leaves of cyclamen were cut into about 1 cm2 (Figure 2A), petiole into 2 cm (Figure 2B) and callus into 0.5 cm3 (Figure 2C), placed on callus induction medium. Incubating in dark for 2 days, detecting the strain amount of Agrobacterium tumefaciens EHA105 under OD600=0.4. Infecting the pre-incubated materials with 100 μmol/L acetosyringone (As) for 6~10 min, and then using sterile filter paper to dry the materials, being transferred into callus induction medium (containing 100 μmol/L As), and cultured under the dark condition for 2~3 days, co-cultured explants were transferred into the selective medium after the delay selective culture for 7 days. The transformed receptor continuingly transferred into the differentiation medium for resistant screening until the calli or adventitious buds differentiated, and finally the clustering seedlings transferred into the rooting medium.
3.6 Optimization of Agrobacterium-mediated genetic transformation method of cyclamen
During leaves being infected by Agrobacterium strains, ultrasonic treatment was accessorially conducted with the power of 100 W for 30 s, 60 s and 120 s (POLYTRON, PT1300D), without ultrasonic treatment as a control. And then co-cultured the medium with 100 μmol/L AS 3 days for co-culture and in delay culture medium for 7 days, prior to selective culture.
3.7 GUS staining for resistant tissues
Taking leaves and calli from resistant plants and wild-type of cyclamen as a control, respectively, which were immersed in freshly prepared X-Gluc dye, soaked 48 h at room temperature or 37℃ for 2 h, destained with 90% ethanol prior to staining observation (Jefferson, 1987).
3.8 Resistant plants by PCR and RT-PCR detection
Genomic DNA and RNA were extracted from resistant plants and wild-type of cyclamen by using CTAB method and Trizol method, resectively. The isolated RNA was reversed to cDNA as a template for PCR amplification. CFL gene-specific primersdesigned as following: Primer F: 5'-GTAGCTGAACGGTACGGTGT-3', Primer R: 5'-ACTAGATACGCATGTC-3', and PCR reaction were conducted in the reaction volume of 25 μL containing dNTP (10 mmol/L) 0.5 μL, 10× Buffer 2.5 μL and performed following as: 94℃ pre-denaturation 4 min and then 30 cycles with 94℃ 30 s, 55℃ 30 s, 72℃ 60 s, finally 72℃ for 10 min extension. PCR products were separated by 0.8% agarose gel electrophoresis, and then scored by gel imaging system (Bio-RAD, Gel Doc).
Authors' contributions
Jing Xu and Xue Han are the persons who conducted this experiment and paper preparation; Xiaogen Pan involved in the data analysis. Jiliang Pang headed this project to make project design, data analysis, and paper revision. All authors had read and consented the final manuscript.
Acknowledgements
This research was sponsored by the National foundation of natureal Sciences (Project No. 31071818); Authors thank for two anonymous reviewers with their critical comments. In this paper we mentioned some chemical and reagent suppliers, which doesn't mean we would like to recommend or endorse the production of theirs.
References
Aida R., Hirose Y., Kishimoto S., and Shibata M., 1999, Agrobacterium tumefaciens-mediated transformation of Cyclamen persicum Mill, Plant Science, 148: 1-7
http://dx.doi.org/10.1016/S0168-9452(99)00072-2
Blazquez M.A., Soowal L.N., Lee I., and Weigel D., 1997, LFY expression and flower initiation in Arabidopsis, Development, 124(19): 3835-3844
Chen L., Zhou L.X., Ma F.W., Liang D., and Hua Z.Y., 2008, Transgenic Cyclamen plants expressing MnSOD gene and its resistance to high temperature stress, Xibei Nonglin Keji Daxue Xeubao (Journal of Northwest A & F University (Nat. Sci. Ed.)), 36(3): 155-160
Dai Y.M., Lin J.B., Wang W.Y., Zou H., Wu S.J., and Lin Y.X., 2010, Transformation of blue gene in Narcissus tazetta var. chinensis mediated by Agrobacterium tumefacien, Nongye Shengwu Jishu Xuebao (Journal of Agricultural Biotechnology), 18(2): 231-238
He Z., Zhu Q., Dabi T., Li D., Weigel D., and Lamb C.J., 2000, Transformation of rice with the Arabidopsis floral regulator LFY causes early heading, Transgenic Research, 9: 223-227 http://dx.doi.org/10.1023/A:1008992719010
Hu J.C., and Liu J.Y., 2008, Developing situation, problems and countermeasures on Cyclamen persicum in China, Beifang Yuanyi (North Horticulturae), (7): 98-101
Jefferson R.A., 1987, Assaying chemric genes in plants: The GUS staining gene fusion system, Molecular Biology Reports, 5: 387-405 http://dx.doi.org/10.1007/BF02667740
Jiang L., Maoka T., Komori S., Fukamachi H., Kato H., and Ogawa K., 2004, An efficient method for sonication assisted Agrobacterium-mediated transformation of coat protein (CP) coding genes into papaya (Carioca papaya L.), Acta Biologiae Exprimentalis Sinica, 37(3): 189-198
Li J., 2004, Studies on the Agrobacterium-mediated cyclamen transformation of chitinase gene, Thesis for M.S., Horticulrure Institute, Northeast Agricultural University, Supervisor: Che D.D., pp.5
Liu F.Q., Zhu G.L., Luo D., Wu X.Y., and Xu Z.H., 1999, Cloning and analysis of CFL-A LFY-like gene from cucumber, Acta Botanica Sinica, 41(8): 813-819
Pena L., Martin-Trillo M., Juarez J., Pina J.A., Navarro L., and Martinez-Zapater J.M., 2001, Constitutive expression of Arabidopsis LFY or APETALA1 genes in citrus reduces their generation time, Nat. Biotech. Nol., 19: 263-267 http://dx.doi.org/10.1038/85719
Shao H.S., Li J.H., Zheng X.Q., and Chen S.F., 1999, Cloning of the LFY cDNA from Arabidopsis thaliana and its transformation to Chrysanthemum morifolium, Zhiwu Xuebao (Acta Botanica Sinica), 41(3): 268-271
Trick H.N., and Finer J.J., 1998, Sonication assisted Agrobacterium-mediated transformation of soybean embrogenic suspension culture tissue, Plant Cell Reports, 17: 482-488 http://dx.doi.org/10.1007/s002990050429
Wang G.L., and Fang H.J., eds., 2002, Plant genetic engineering, Second Edition, Science Press, Beijing, China, pp.464-465
Wang L.L., Liang H.M., Pang J.L., and Zhu M.Y., 2004, Regulation network and biological roles of LEAFY in Arabidopsist haliana in floral development, Yichuan (Hereditas), 26 (1): 137-142
Weigel D., and Coupland G., 1995, LEAFY blooms in aspen, Nature, 377: 482-483 http://dx.doi.org/10.1038/377482a0
Yao C.N., and Wang Y.F., 2001, Sonication-assisted Agrobacterium-mediated transformation of cucumber, Yuanyi Xuebao (Acta Horticulturae Sinica), 28(1): 80-82
Zhao L.J., Liu W.L., Li D.Y., Sun A.Q., and Zhao X.Q., 1995, Current progress in Cyelamen research, in: Han Z.H., Huang W.D., and Xu X.F. (eds.), The proceedings of the secondly youth annual conference of china association for science and technology, Horticulture Dissertation, Beijing Agriculture University Press, Beijing, China, pp.599-607
Zhang M.Z., Ye D., Wang L.L., Pang J.L., Zhang Y.H., Zheng K., Bian H.W., Han N., Pan J.W., Wang J.H., and Zhu M .Y., 2008, Overexpression of the cucumber LEAFY homolog CFL and hormone treatments alter flower development in gloxinia (Sinningia speciosa), Plant Mol. Biol., 67: 419-427 http://dx.doi.org/10.1007/s11103-008-9330-8
Zhao W.L., Jiang S.P., Fu X.S., Zhu Y.L., and Yang K.S., 2005, Introduction of Chalcone Synthase-A (CHSA) gene into Cyclamen persicum via pollen-tube Pathway, Molecular Plant Breeding, 3(4): 531-536
Zhu Y.L., and Ma F.W., 2009, Establishment of Agrobacterium-mediated transformation system for Cyclamen persicum, Xibei Nongye Xuebao (Acta Agriculturae Boreali-occidentalis Sinica), 18(3): 240-244
. PDF(514KB)
. HTML
Associated material
. Readers' comments
Other articles by authors
. Jing Xu
. Xue Han
. Xiaogen Pan
. Jiliang Pang
Related articles
. Cyclamen persicum
. Cucumber flowering integrator
. CFL gene
. Agrobacterium -mediated transformation
. Sonication-assisted transformation
Tools
. Email to a friend
. Post a comment


